6.3 Gibbs Free Energy
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What are some examples of spontaneous processes in everyday events?
Ice Melting
Hot food cooling
Metal Rusting
What makes everyday processes like ice melting or metal rusting spontaneous?
These events occur naturally without external help because they allow energy to spread out more evenly, and energy likes to spread out instead of staying packed in one place, leading to an increase of entropy.
What is the Second Law of Thermodynamics?
Energy spontaneously disperses, if not hindered
When energy disperses, entropy increases in the combination of system plus surroundings
What happens to the entropy of the universe over time?
It always increases, things naturally become more spread out and disordered
What makes a reaction spontaneous?
It’s spontaneous if the total entropy (system+ surroundings) goes up
This is written as ΔSₜₒₜ > 0, which means the total disorder goes up
Can we measure the entropy of the whole universe?
No, it is too big but scientists can estimate it by looking at energy and temperature changes
What does a reaction’s spontaneity depend on?
How the reaction changes in disorder (system)
How the surroundings change in disorder or energy
What does the equation ΔSₛᵤᵣᵣ = −ΔHₛyₛ / T mean?
The change in disorder (entropy) of the surroundings depends on how much heat the reaction gives off or taken in, and on the temperature
If the reaction releases heat → surroundings entropy increases
If the reaction absorbs heat→ surroundings entropy decreases
The effect is smaller in higher temperatures
What is the Gibb’s free energy equation and what does it tell us?
ΔG=ΔH−TΔS
It shows if a reaction will happen on its own:
ΔG < 0 → spontaneous
ΔG > 0 → not spontaneous
ΔG = 0 → at equilibrium
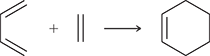
Explain the ΔS reaction and ΔH
two molecules combine into one and stronger bonds are formed — here’s how it works:
Entropy (ΔS) → Negative (–)
Because the reaction goes from two molecules to one, things become more organized (less random). Nature prefers disorder, so this is unfavorable.Enthalpy (ΔH) → Negative (–), not positive!
Because the reaction forms stronger σ bonds and releases heat, it’s exothermic, meaning ΔH is negative.
So together:
✅ ΔS = – (unfavorable)
✅ ΔH = – (favorable / exothermic)
When is a reaction spontaneous?
A reaction is spontaneous when when ΔG is negative.
This means the reaction happens on its own
What do the two parts of the Gibbs equation (ΔG = ΔH − TΔS) mean?
ΔH → heat term (favorable if negative, releases heat)
−TΔS → entropy term (favorable if entropy increases, ΔS positive).
The two terms compete to decide if ΔG is negative or positive.
How does temperature affect spontaneity?
The TΔS part depends on temperature.
Low T: ΔH (heat) dominates → reaction may be spontaneous.
High T: TΔS dominates → reaction may become nonspontaneous.
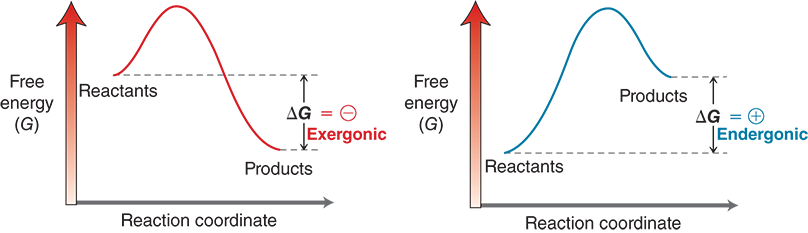
What are exergonic and endergonic reactions?
Exergonic (ΔG < 0): energy is released → spontaneous.
Endergonic (ΔG > 0): energy is absorbed → not spontaneous.
What do energy diagrams show?
They show how energy changes during a reaction, helping us see:
The activation energy needed to start it.
Whether it releases or absorbs energy overall.