Blood Coagulation 2
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
Arterial Thrombosis
What is it usually as a consequence of?
Atherosclerosis
Arterial Thrombosis
Major associated problems
Heart attack
Stroke
Arterial Thrombosis
Key aims in prevention
Remove / reduce modifiable risk factors e.g. smoking, hypertension, diabetes, lack of activity obesity
Bear in mind unavoidable factors e.g. age, sex, family history
What are Acute Coronary Syndromes?
A range of acute myocardial ischaemic states
Includes
Unstable angina
NSTEMI
STEMI
What is atherosclerosis?
Build up of plaque (atheroma) between the arterial intima and basement membrane
Caused by a chronic inflammatory process » engulfment of cholesterol by macrophages, forms foam cells which form the atheroma
Lumen becomes narrowed
Which reduces blood flow
2 types of atherosclerotic plaque
Hard and stable
Fibrous cap over the atheroma
Less problematic
Soft and unstable
Problematic
Why are soft and unstable atherosclerotic plaques problematic?
When exposed to the shear forces within the blood vessel, they are more prone to:
Being ruptured
Breaking off
Exposing the underlying the basement membrane = causes thrombosis within the blood vessel
What happens during a myocardial infarction?
Rupture of an atheromatous plaque
Causes development of a thrombus » platelet aggregation, fibrin deposition and activation of inflammatory mediators
Total occlusion of coronary artery
Ischaemia (no blood flow)
Thrombolysis
What is Thrombolysis?
Used in myocardial infarction
Aims to re-establish coronary blood flow to:
Reduces myocardial damage
Preserves LV function
Thrombolysis
How soon should Thrombolysis be established?
’Time is muscle’
Should give as soon as onset of pain is possible
Ideally within a ‘golden hour’
Thrombolysis
Contraindications
Risk of bleeding so:
Recent major injury
Peptic ulcer
Risk of subarachnoid haemorrhage
Recent head injury
Prolonged and vigorous CPR
Thrombolysis
Why does thombolysis increase risk of bleeding?
Leads to fibrin clot being dissolved
So reduces blood clotting = increases risk of bleeding
Interventional Cardiology
What is interventional cardiology?
PCI (Percutaneous Coronary Intervention)
Interventional Cardiology
Is it preferred to Thrombolysis in the treatment of myocardial infarction?
YES
Mechanically improves myocardial perfusion
So reduces risk of bleeding
Interventional Cardiology
Advantages
Mechanically improves/restores myocardial perfusion
Better outcomes than thrombolysis in treatment of MI
Lacks the haemorrhagic risks of thrombolysis
Can be used when thrombolysis is contraindicated
Stents can be inserted to help keep arteries patent
Antiplatelet drugs are given before, during and after
Ischaemic Stroke
What is ischaemic stroke?
Occlusion of the arteries supplying blood to the brain
Ischaemic Stroke
Approx. 85% of strokes are ischaemic. What are the other 15% classified as?
Haemorrhagic » due to a bleed in the brain
Ischaemic Stroke
Most common type of ischaemic stroke
What drugs are used for the prophylaxis of this type of ischaemic stroke?
Thrombotic stroke
Thrombosis of a cerebral artery (artery supplying the brain)
Due to atherosclerosis
Prophylaxis:
Anti-platelet drugs » to prevent formation of thrombus
Ischaemic Stroke
Less common type of ischaemic stroke
What drugs are used for the prophylaxis of this type of ischaemic stroke?
Embolic stroke
Thrombus originates in the heart and embolises in the brain = blocks an artery in the brain which reduces blood flow
Strong association with AF, recent MI and valve dysfunction » where there is a disruption of organised flow of blood through the heart = stagnant blood = formation of thrombus
Prophylaxis:
Anticoagulant drugs » As problem is due to stagnant blood forming a fibrin-based clot
Ischaemic Stroke
What do the signs and symptoms associated with ischaemic stroke depend on?
The size of the blocked vessel
The location of the blocked vessel
Ischaemic Stroke
Where will motor symptoms associated with stroke occur?
On the opposite side of the body to the side of the affected vessel
Ischaemic Stroke
Signs and Symptoms of stroke
Facial weakness: Can the person smile? Has their mouth or eye drooped?
Arm weakness: Can the person raise both of their arms?
Speech problems: Can the person speak clearly and understand you?
Time to call 999: Any of the above signs
Ischaemic Stroke
Which type of ischaemic stroke tends to lead to more severe outcomes and why?
Embolic stroke
The size of the artery blocked tends to be larger
Venous Thrombosis
What is venous thrombosis?
Thrombus which develops in the deep veins e.g. lower leg
AKA Deep Vein Thrombosis (DVT)
Venous Thrombosis
What is the major concern with venous thrombosis?
Pulmonary embolism
Risk that thrombus will break off and emobilise
Carried through the venous circulation to the heart and pulmonary circulation = blocks pulmonary artery with a narrower diameter than the clot
Post-phlebitic syndrome
Inflammation in blood vessels affected by venous thrombosis
Painful for patient
Venous Thrombosis
Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE) comprise a single clinical entity:
Venous Thromboembolism (VTE)
Venous Thrombosis
What is meant when we say ‘Management of VTE’?
Management of BOTH DVT and PE
Venous Thrombosis
Risk factors of venous thrombosis
Age
Immobility
Recent operation esp in the legs or hips
Pregnancy
Oral contraceptive
HRT
Cancer and chemotherapy
Travel
Family history
Genetic thrombophilia
Venous Thrombosis
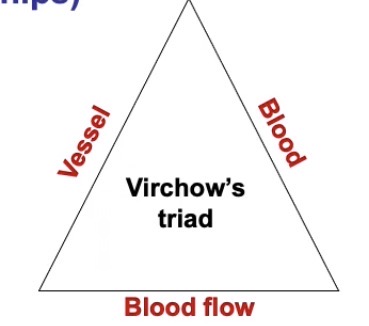
What does Virchow’s Triad show?
An accumulation of risk factors for venous thrombosis
It describes:
Issues with the blood vessel
Issues with the quality of the blood
Issues with the flow of blood
» If even one factor is disrupted, can cause venous thrombosis
» If more than one factor is disrupted, significantly increases risk
Venous Thrombosis
Risk Factors
Immobility
Stagnation of venous blood flow
= Formation of thrombus
Venous Thrombosis
Risk Factors
Recent operation esp in the legs or hips
Immobility = stagnant blood
Activation of tissue factor pathway due to surgery = activation of coagulation mechanisms
Venous Thrombosis
Risk Factors
Age
Considered an independent AND a non-independent risk factor:
Independent risk factor:
Quality of blood vessels and CVS declines with age
Non-independent risk factor:
Older individuals are more at risk of falls, operations, HRT, cancer etc
Venous Thrombosis
Risk Factors
Pregnancy
Immobility
Changes in levels of fibrinogen and other coagulation factors e.g. factor 8 = affects blood and blood flow in Virchow’s Triad
» Whilst there is a risk of venous thrombosis in pregnancy, not a major problem unless patient already has risk factors before pregnancy
Venous Thrombosis
Risk Factors
Travel
Immobility = stagnant blood
Venous Thrombosis
Risk Factors
Genetic thrombophilia
Genetic factors that affect the ability of the blood to clot
Affects the blood side of Virchow’s Triad
Venous Thromboembolism
Signs and symptoms of VTE
DVT: pain, swelling and heat in affected limb
PE: acute chest pain, dyspnoea
Venous Thromboembolism
What does the severity of PE depend on?
The size of the vessel affected
Venous Thromboembolism
What is a ‘silent’ PE?
Such a small vessel is affected that patient is unaffected
Venous Thrombolemolism
Treatment of VTE
Anticoagulant drugs » inhibit plasma coagulation
Heparin
Warfarin
DOACs
Thrombolysis or surgical removal of thrombus (thrombectomy) only in severe cases
Warfarin
Overall mechanism of action
Vitamin K antagonist
Inhibits synthesis of factors X, IX, VIII and II in the liver » involved in plasma coagulation
By preventing the conversion of non-functional factors to functional factors via post-translational modification
So they are released into the blood in a non-functional form
Warfarin
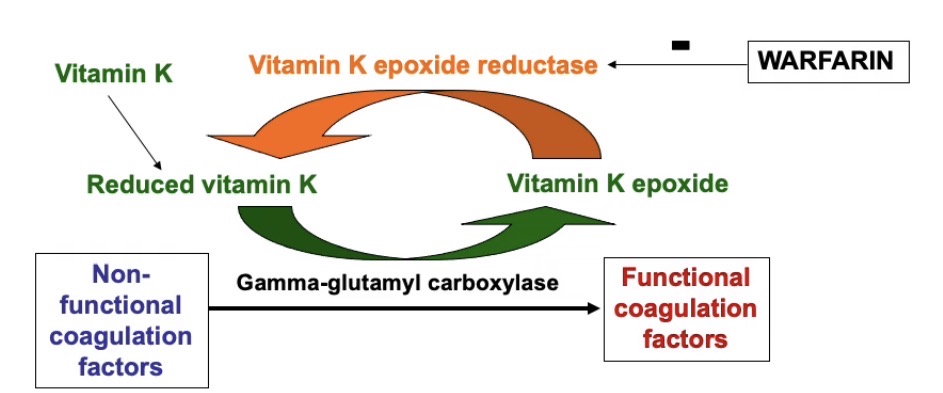
How are non-functional coagulation factors converted to functional coagulation factors and how does warfarin inhibit this to have an anticoagulation affect?
Non-functional coagulation factors are converted into functional coagulation factors by an enzyme called gamma glutamyl carboxylase
This enzyme carboxylates glutamic acid residues on the non-functional coagulation factors
Which allows them to bind to calcium and phospholipids when they enter the blood
Which allows the non-functional factors to be converted to their functional form
Gamma glutamyl carboxylase carries out its reaction with a paired reaction of the oxidation of vitamin K to vitamin K epoxide
Vitamin K epoxide reductase recycles the Vitamin K back to its reduced form, so it can take part in the reaction again
Warfarin inhibits the Vitamin K reductase = indirectly inhibits action of gamma glutamyl carboxylase = prevents conversion of coagulation factors into their functional form
Warfarin
What is warfarin a derivative of?
Synthetic coumarin derivative
Dicoumarol found in mouldy sweet-clover hay
Causes a bleeding disorder in cows
Warfarin
How is warfarin given?
As a racemic mixture
S-warfarin 5x more potent than R-warfarin as an anticoagulant
BUT
S-warfarin (CYP2C9) metabolised more rapidly than R-warfarin (CYP3A4)
Warfarin
Problems with warfarin
Many drug and food interactions
Narrow therapeutic index
Unwanted bleeding
Patient must keep diet and vitamin K stable » can affect efficacy of warfarin
INR monitoring required
Warfarin
Drug interactions
Drugs that increase warfarin metabolism reduce anticoagulant effect e.g. carbamazepine, primidone, phenytoin, rifamycins
Drugs that reduce warfarin metabolism increase anticoagulant effect e.g. cimetidine, amiodarone
Highly albumin bound drugs may increase anticoagulant effect e.g. NSAIDs
Use of antiplatelets (e.g. aspirin) + warfarin increases risk of bleeding
Warfarin
Warfarin + Anti-epileptic drugs
Increase warfarin metabolism by inducing liver enzymes
Reduces anticoagulant effect of warfarin
E.g. carbamazepine, phenytoin
Warfarin
Warfarin only works…
In vivo
Warfarin
How long does it take for anticoagulant effect to be achieved?
Takes several days for full anticoagulant effect to be achieved and stabilised
So patient may need to be on another anti-coagulant e.g. heparin that works immediately in the meantime
Warfarin
Why does warfarin take several days to work?
The coagulation factors involved (2, 7, 9 and 10) will need to be replaced by new non-functional coagulation factors synthesised in the presence of warfarin
The rate at which each factor is replaced varies = takes several days to stabilise
Warfarin
Antidote in the case of warfarin overdose
Phytomenadione (Vitamin K1)
» Replenishes Vitamin K in the liver should the effects of warfarin needs to be reversed
Heparin
Where does it come from?
Naturally occurring molecule in the body
Heparin
Structure
Glycosaminoglycan
Formed from repeating disaccharide units
Extensively N and O sulfated = very negatively charged molecule
Heparin is the most negatively charged molecule that exists in nature
Heparin
Mechanism of action
Potentiates the activity of the body’s main natural anticoagulation inhibitor, antithrombin:
Antithrombin inhibits the activity of thrombin (factor 2a) and factor 10a
Antithrombin is a serpin (serine protease inhibitor)
Heparin binds to antithrombin
The reactive arginine centre of the antithrombin becomes more accessible to the serine protease, so it is more readily able to inhibit it in factor 2a and 10a
So heparin accelerates the rate at which antithrombin is able to inhibit coagulation factors
Heparin
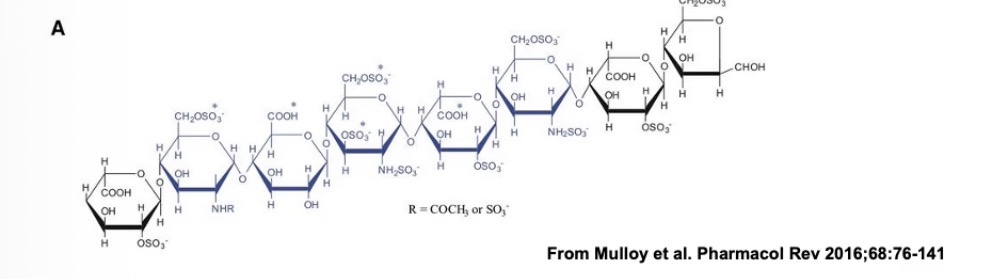
Which part of heparin binds to the antithrombin?
Pentasaccharide region (blue)
Heparin
Where is heparin found in the body?
inside mast cells
Heparin
Which 2 preparations of heparin are available?
Unfractionated heparin
Low molecular weight heparin (LMWH)
Heparin
What is the difference between unfractionated heparin and LMWH?
Unfractionated heparin: heterogeneous mixture of long and short chains of heparin
LMWH: homogenous mixture of low molecular weight chains
Heparin
Advantages of LMWH over unfractionated heparin?
Longer half life = administered less frequently
Heparin
Advantages of unfractionated heparin over LMWH?
More reversible
Heparin
What is fondaparinux and what is its advantage?
The pentasaccharide sequence of heparin is enough to potentiate the inhibitory action of antithrombin on factor 10a
Whereas a longer sequence is needed to increase the inhibitors of thrombin (factor 2a) by antithrombin
Fondaparinux is a synthetic antithrombin binding pentasaccharide
Able to inhibit factor 10a but has no affect on thrombin (2a)
Heparin
Heparin is an…
Indirect anticoagulant » as exerts activity via antithrombin
Heparin
Heparin works both…
In vivo AND in vitro
(If you add heparin to blood in a test tube, it would not clot, whereas if you added warfarin to blood in a test tube it would still clot)
Heparin
How must heparin be given?
By injection
Heparin
How long does it take to work?
Immediate anticoagulant effect » because all the components it needs to work are already in the blood
Heparin
Disadvantages
Risk of bleeding
Serious hypersensitivity reactions
Physical incompatibility with many drugs
Heparin
Example of hypersensitivity reaction to heparin
Heparin induced thrombocytopenia
Occurs on repeated administration of heparin over a number of days
Antibodies are created against a protein associated with platelets
Causes destruction of platelets
Heparin
Reversal agent
Protamine
Effective against unfractionated heparin
Less effective against LMWHs
Heparin
When is heparin used?
Prophylaxis against VTE after surgery
Can be used in pregnancy » warfarin is contraindicated as teratogenic
Used while warfarin effects become established
Can anticoagulants e.g. warfarin and heparin be used to treat an existing clot/thrombus?
YES
What is hirudin?
Peptide found in salivary gland of leeches
Anticoagulant
Directly inhibits thrombin
So allows leeches to extract blood
Which drugs were modelled on hirudin?
When are these drugs sued?
Leperudin: no longer marketed
Bivalirubin: peptide, direct thrombin inhibitor
Argatroban: small molecule, direct thrombin inhibitor
Given via infusion
Useful alternatives if patient cannot receive heparin