9. Organic Chemistry Basics
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
94 Terms
What to know for organic chemistry questions:
Nomenclature
Structure/Behavior
Key Reactions
Lab Techniques
Alkanes
Simple hydrocarbons composed of only single bonds
How to name alkanes:
Identify/name longest chain (parent chain)
Label substituents so they have the lowest numbers
Name substituents with -yl suffix
Di, tri, tetra for multiple of the same substituents
Iso and cyclo count towards alphabet
If multiple paths, use one with MORE substituents
Functional Group
Group of atoms/bonds responsible for a characteristic set of behaviors (Alcohols, amines, etc.)
Naming with functional groups:
Find parent chain but chain molecule ending to functional group’s specific ending
If more than 2 functional groups, pick highest priority
Functional Group Naming Priorities:
Carboxylic Acid > Ester > Acid Chloride > Amide > Aldehyde > Ketone > Alcohol > Thiol > Amine > Alkyne > Alkene > Alkane
Alkyl halides and ethers are lower than alkane
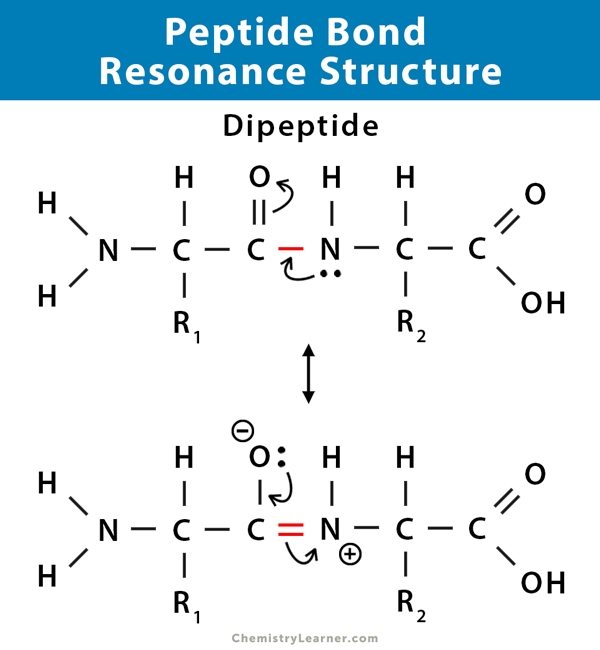
Resonance
Equivalent structures in which electrons are delocalized
Electron Delocalization
Result of resonance that affects chemical properties and contribute to a molecule’s stability
Common Structures with Resonance on MCAT
Carboxylate anion, Peptide Bond
Conjugation
Form of resonance when three or more adjacent p-orbitals align (conjugated double bonds); π system
How to tell if a molecule is conjugated?
Alternating sigma and pi bonds
What spectrum are conjugated systems detected by?
UV-VIS (utilizes low EM energy)
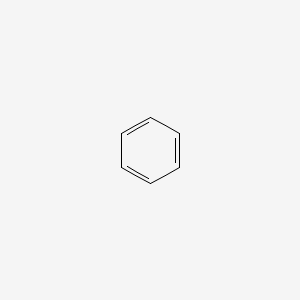
Aromatic Compounds
Conjugated, cyclic, planar molecules that follow Huckel’s Rule (4n+2)
How to tell if a compound is aromatic?
Has a conjugated system, is planar, and followed 4n+2 (if it follows 4n rule, it is antiaromatic)
Benzene
Pyridine
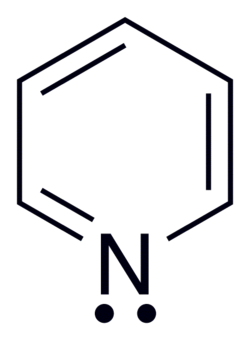
Pyrimidine
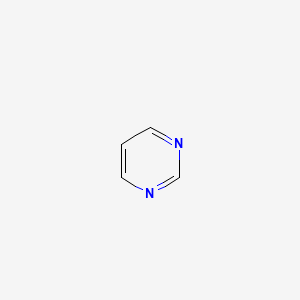
Purine
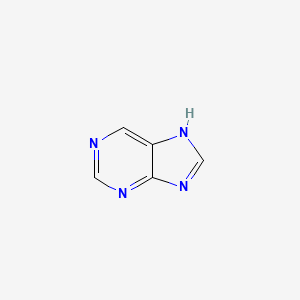
Imidazole
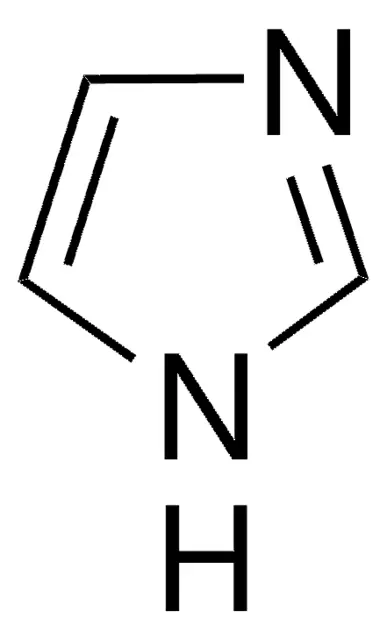
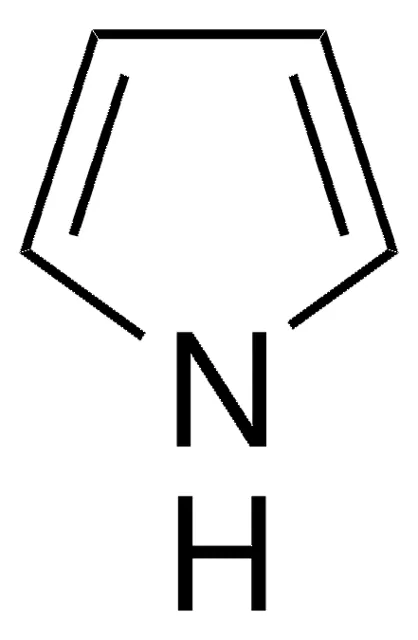
Pyrrole
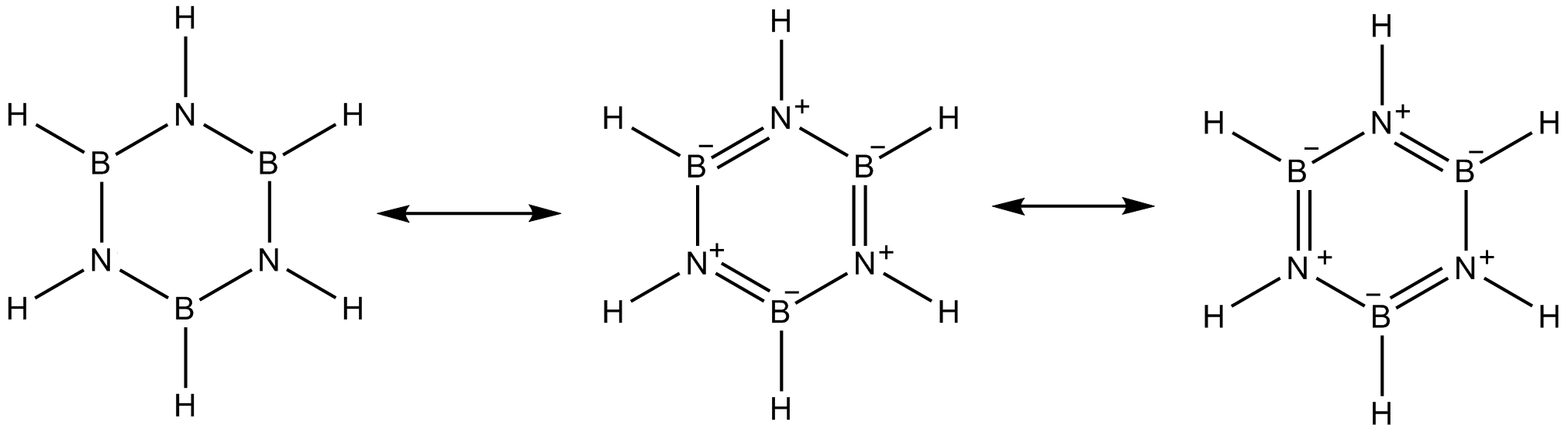
Borazine
Heterocycles
Aromatic rings containing atoms other than carbon
What is pyridine found in?
NiacinWha
What purine and pyrimidine found in?
Nucleic acids
How does resonance affect bond lengths?
Causes bonds to be intermediates of double and single bonds
Heat of Hydrogenation
How much energy would be released upon adding hydrogens to double bonds; Exothermic
Heat of Hydrogenation =
ΔHo bonds broken - ΔHo bonds formed
(ΔHo x # of pi bonds, negative)
Why does benzene have a lower heat of hydrogenation than cyclohexene?
Benzene is resonance stabilized, so it has lower internal energy to begin with (more stable)
Heat of Hydrogenation Trend
Decreases with resonance stability
Decreases with more substituents (mono vs di vs tri-substituted)
Cis is higher than trans
Why is phenol more acidic than a regular alcohol?
If the phenol loses a hydrogen, there is resonance stability so it is more likely to donate a proton.
Structural/Constitutional Isomers
Same molecular formula but different structure (way their constituents are connected)
Should have different names but same # atoms
Stereochemistry
How molecules are arranged in space
Functional Isomers
Structural isomers with different functional groups
How to tell if two molecules are structural isomers?
Make sure there is different connectivity of substituents BUT have same formula (count # of molecules)
Tautomers
Structural Isomers that interconvert with each other, in equilibrium
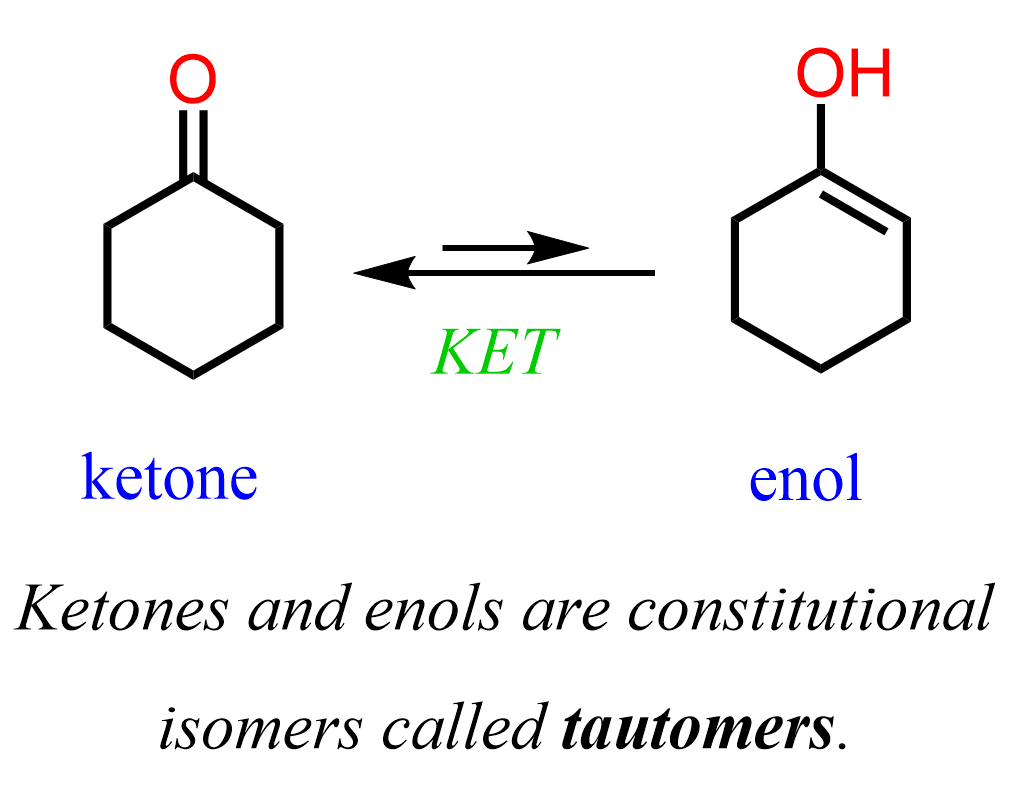
Keto-enol tautomerism
Keto-enol Tautomerism
Keto formed favored, enol form is a double bond and hydroxyl group with a enolate ion intermediate for reactions
Resonance vs. Tautomers
Resonance are the SAME structures with delocalized electrons
Tautomers are DIFFERENT structures due to breaking/forming bonds
Examples of Tautomers
Keto and enol
Enamine and imine
Lactams and lactims
Amids and amidic acids
Stereoisomers
Molecules with one pattern of connectivity have different spatial arrangement
3 ways Stereochemistry occurs:
Arrangement of single bonds (rotation)
Orientation (cis or trans) across double bond
Orientation at chiral center (R/S, d/l system)
Can double bonds be rotated?
No, they are locked in either cis or trans; rotating would break bond
Newman projections
Visualize orientation around a single bond; front carbon is a dot, back carbon is a circle
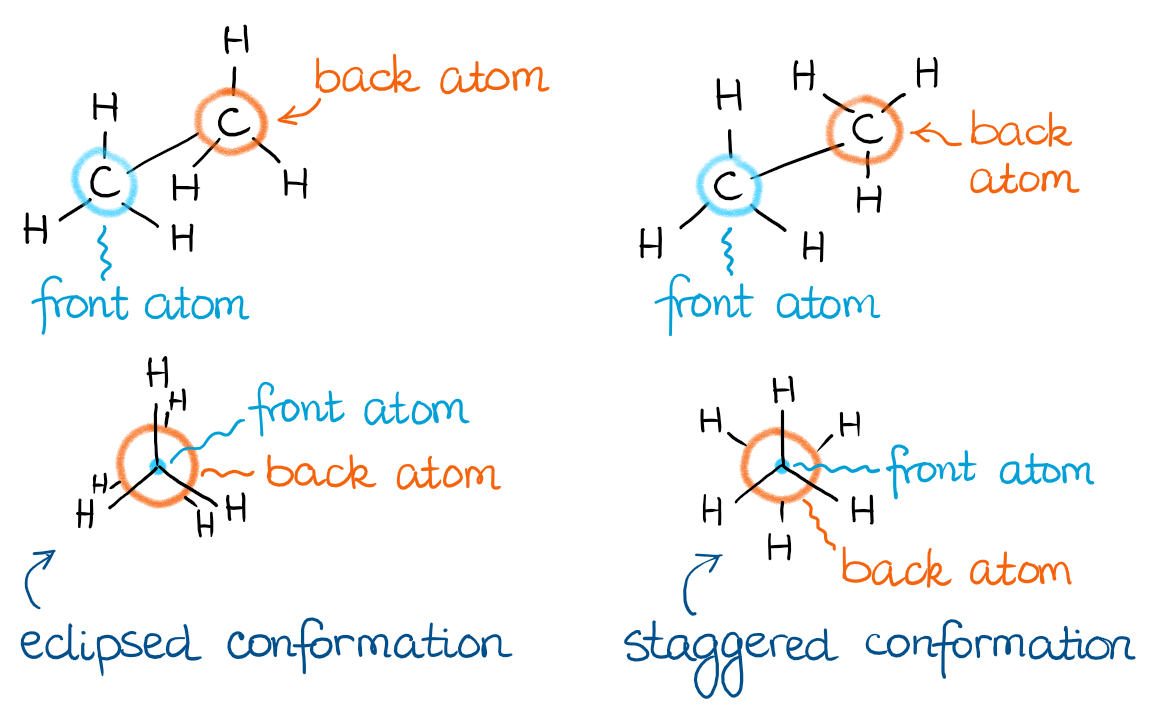
Eclipsed (close) vs. Staggered
Eclipsed is more unstable and higher energy due to torsional strain
Staggered Newman Subsets
Anti
Gauche
Eclipsed Newman Subsets
Eclipsed
Totally eclipsed
Anti Newman
Most stable, least torsional strain; maximum separation of 180o
Gauche Newman
Staggered but closer together with 60o separation
Eclipsed Newman
Contain overlapping orbitals (not the bulkiest ones)
Totally Eclipsed
Bulky groups overlap each other
What type of isomer does rotating around a single bond generate?
Conformational isomers
Which Newman projection orientation has the highest PE?
Total eclipsed because there is the most torsional strain
What is the ideal bond angle?
Tetrahedral, sp3 109.5o angle
Conformational Isomers of Cyclohexanes
Boat, twist-boat, and chair; cyclohexane alternates but chair is most stable
Why does cyclohexane have isomers?
To resolve steric, torsional, and angle strain
Angle strain
Angle between single-bonded carbons deviates from 109.5
Torsional Strain
Eclipsing substituents on neighboring atoms (Newman)
Steric Strain
Substituents (including hydrogen) are too close together
What orientation in the chair do bulky substituents go on?
The equatorial orientation, where repulsions are minimum
Cis cycloalkane
Two substituents that point up or down together

Trans cycloalkane
Two substituents up and down

Can free rotation around C-C bond occur in cycloalkane?
No, there is too much strain in a cyclic molecule.
Cis double bond
Both substituents are on the same side of a double bond
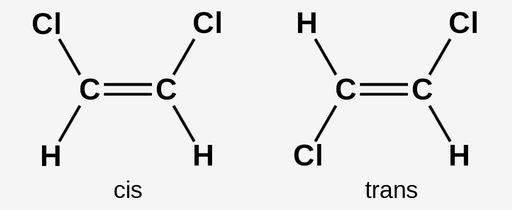
Trans double bond
Substituents are on opposite sides (diagonal) of a double bond
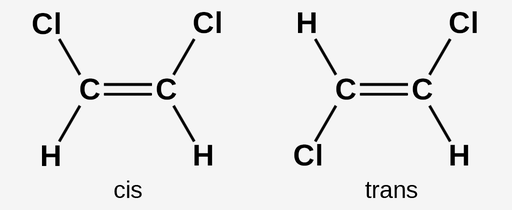
How are fatty acids often described?
Cis or trans (for unsaturated because they contain double bonds)
Which fatty acids contain double bonds?
Unsaturated fatty acids (saturated only have single bonds)
Which unsaturated fatty acids are naturally occuring?
Cis because it prevents them from evenly stacking (trans causing health problems because of stacking)
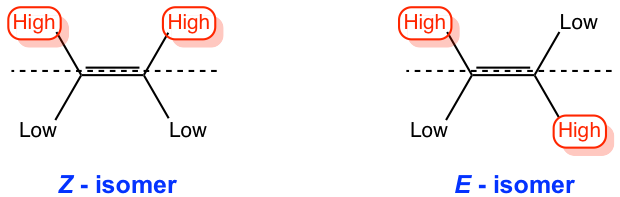
E-Z Classification of Double Bonds
Based on Cahn-Ingold-Prelog (highest) priority of substituents on the double bond (Z or E)
Z-Isomers
Highest priority substituents are on the same side of double bond
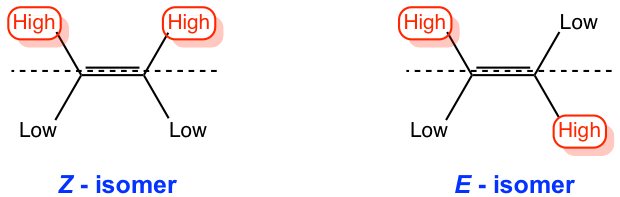
E-Isomers
Highest priority substituents are on opposite sides of double bond
All cis-isomers are __-isomers and all trans-isomers are __-isomers
Z; E
Cahn-Ingold-Prelog Rule
E and Z classification based on atomic weights of substituents or the atomic weight of the atoms attached to substituents if the same
Chiral Centers (Stereocenter)
Atoms connected to an sp3 carbon with four unique groups; non-superimposable on mirror image
Are all stereocenters chiral centers?
No, a stereocenter can be achiral (like a E/Z double bond); BUT a chiral center is ALWAYS a stereocenter
Maximum number of stereoisomers =
2n, n = number of stereocenters
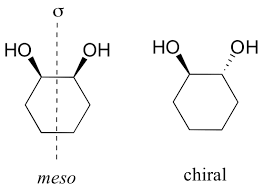
Chiral (enantiomers) vs. achiral molecules
Achiral molecules have a plane of symmetry and CAN be superimposed while enantiomers can not
Meso Compounds
Molecules that MUST have multiple stereocenters (usually 2) with a plane of symmetry, causing them to be achiral
Specific Rotation (l or d), [a]
Solutions of chiral compounds rotate planes of polarized light at unique angles (because of a mix)
Dextrorotary (d)
Clockwise (+) rotation of polarized light
Levorotary (l)
Counterclockwise (-) rotation of polarized light
What are most amino acids’ specific rotations?
Levorotatory (l); except for Cysteine
Cysteine’s R/S Configuration
R (due to thiol -SH group); all other amino acids are S
Specific Rotation Formula [a] =
a/cl
a = observed rotation (degrees)
c = concentration (g/mL)
l = length of tube (dm)
Racemic Mixture
50:50 mixture of stereoisomers; no rotation of light because +[a] and -[a] cancels out
Amino Acid Chirality
All amino acids are chiral except for glycine
% Enantiomeric Excess =
[a]observed/[a]pure
Only how much one exceeds the other (otherwise would be racemic)
R/S System
Follows Cahn-Ingold Rule to determine absolute configuration of stereocenters
How to determine R/S:
Assign substituent priority based off atomic weight
Draw lowest substituent in the back
Order of substituents 1-4 is left to right = R, right to left is S
If lowest priority is in the front, true orientation is opposite
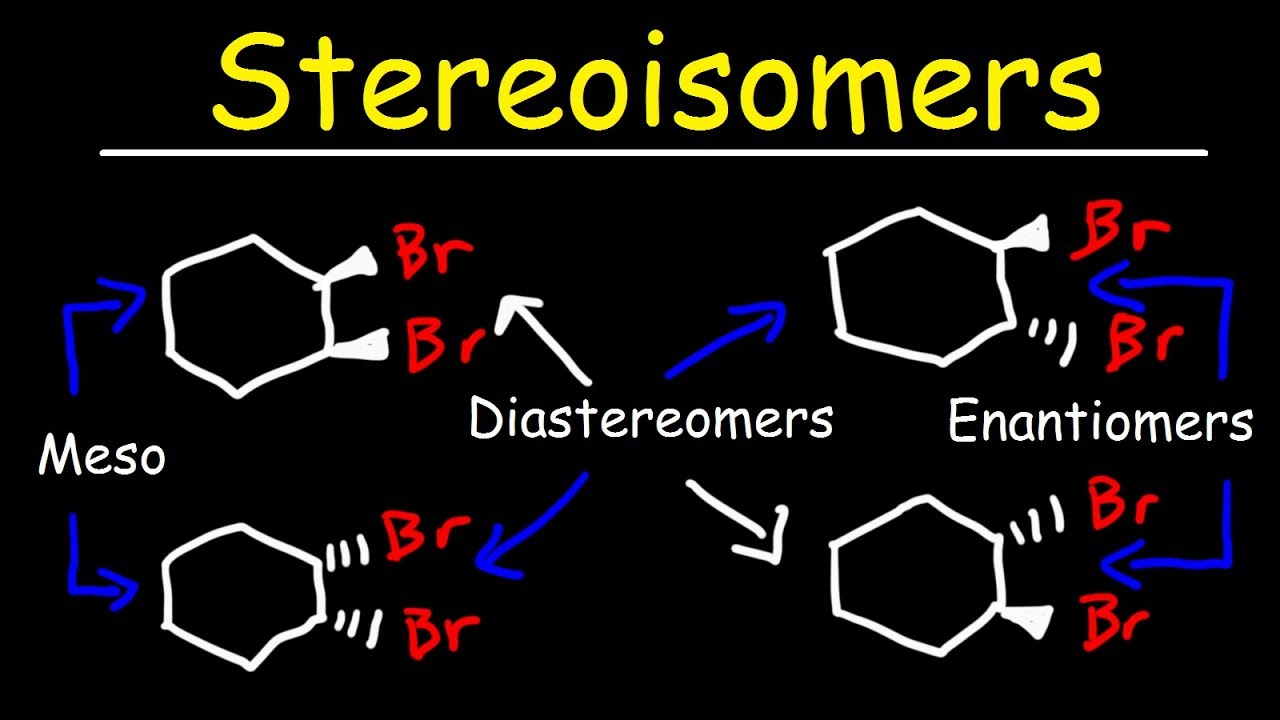
Enantiomers and Diastereomers
Distinctions between two isomers of the same molecule (all stereocenters orientation different or some the same)
Enantiomers
Non-superimposable mirror images; all stereocenters are orientated differently
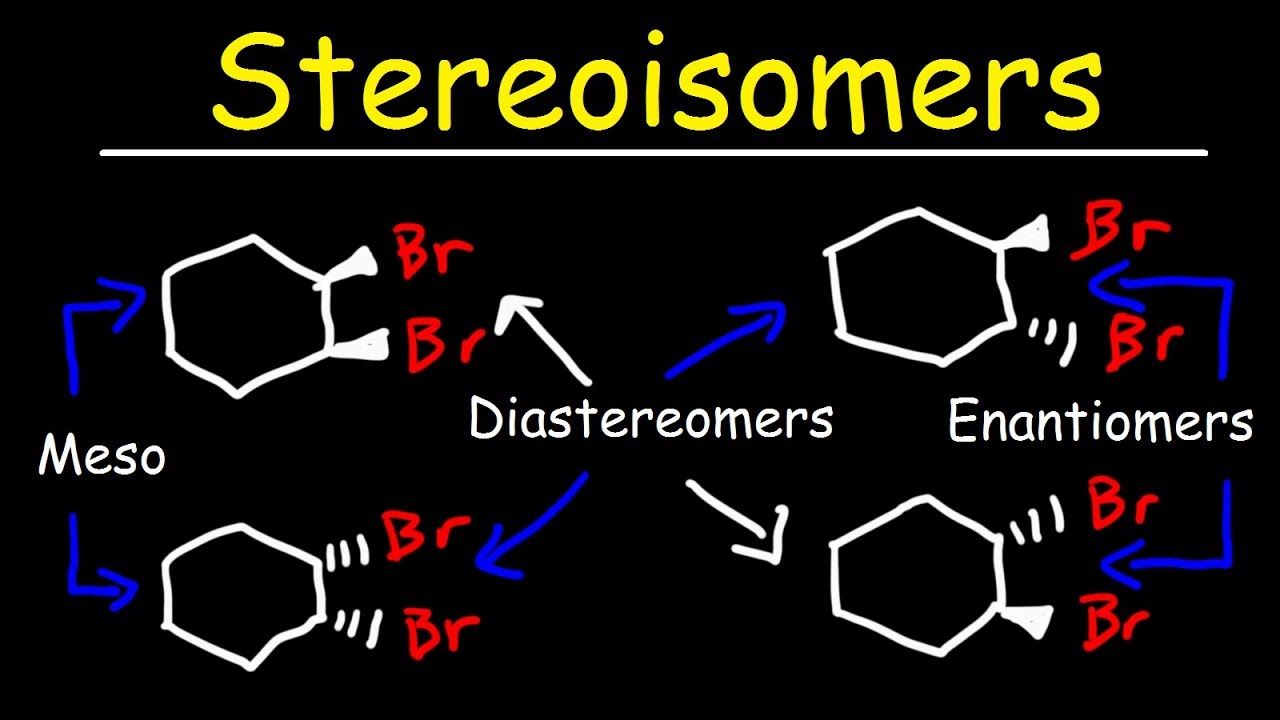
Diastereomers
Not all stereocenters are orientated differently (some are the same); NOT mirror images
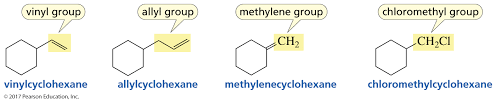
Vinyl Group
Group attached directly to double bond
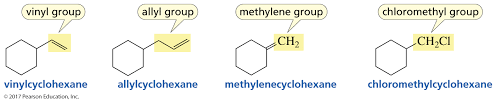
Allylic Group
Group attached adjacent to double bond
Geminal Groups (usually diols)
Groups on the same carbon
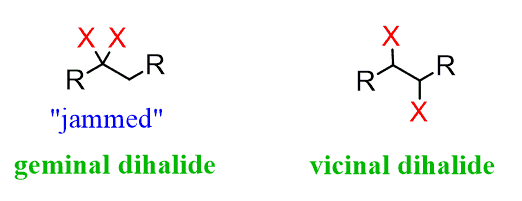
Vicinal Groups
Groups on adjacent carbons