Electrolysis of Aluminium Oxide
1/9
Earn XP
Description and Tags
https://www.youtube.com/watch?v=YcyMElBEzAY&list=PL9IouNCPbCxXDlRtCQEG0cGehBvJ7t9Pf&index=15
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
How do we extract metals
By using reactivity series, we can determine which metals can be extracted by reduction with carbon, or extracted using electrolysis.

How is aluminium extracted
Extracted from aluminium oxide by electrolysis.
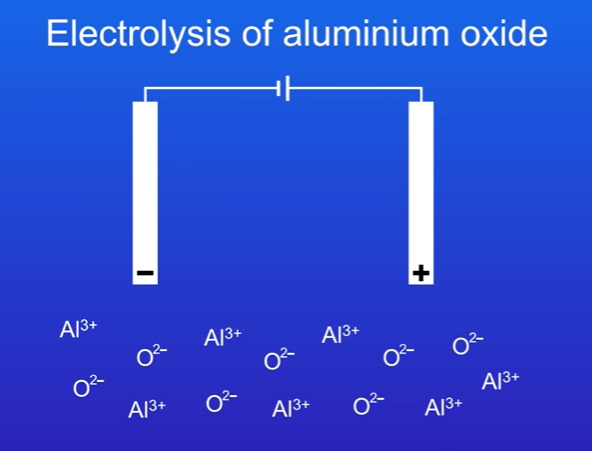
What is aluminium oxide
It contains aluminium ion (Al3+) and the oxide ion (O2-)
It has a very high melting point of 2000 degrees.
Explain first step of electrolysis of aluminium oxide
Its first mixed with cryolite, which lowers the melting point
This reduces amount of energy needed and saves money
Whats second step .
We then apply an electric current to the molten aluminium oxide.
The cathode and anode are made of graphite (carbon).
The ions are free to move.
Why is graphite used.
Its a good conductor of electricity. It has high melting point so it can be used at high temps without it melting.
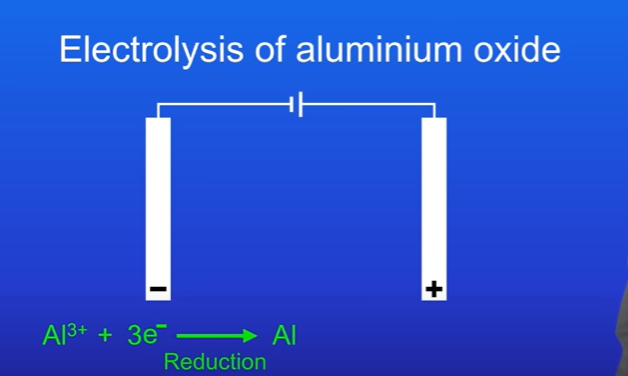
What happens at the cathode
The Al3+ ions are attracted to the cathode ( negative electrode). Each ion gains three electrons and forms an aluminium atom, this is reduction as the aluminium gains electrons.
The half equation must be memorised. Al3+ + 3e- → Al
What happens at the anode
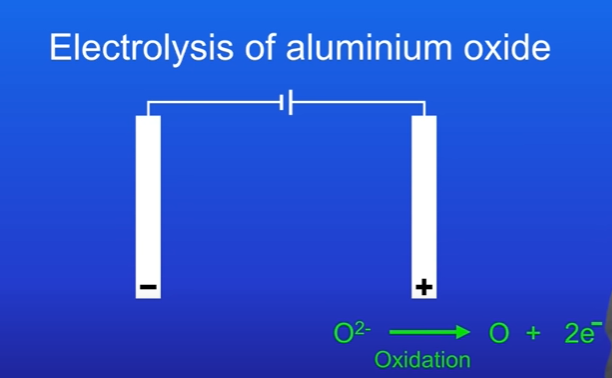
The O2- ions are attracted to the positive terminal ( anode)
Each oxide ion loses 2 electrons to form an oxygen atom.
The oxide ions are losing electrons, so this is a oxidation reaction. The half equation that must be memorised is O2- → O + 2e-
Whats a key point about anode
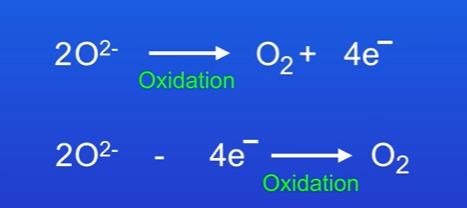
There are 2 versions of the half equation that show the same thing.
Oxygen atoms formed at the anode pair up to form the oxygen molecule O2, so the equations must be doubled.T
The anode must be replaced regularly.
The oxygen molecules produced at the anode react with the graphite (form of carbon) , and forms carbon dioxide.
Why is electrolysis expensive
Melting the compounds such as aluminium oxide requires a great deal of energy.
A lot of energy is required to produce the electric current.