Addition Reactions (markovnikov/anti-markovnikov and syn/anti)
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
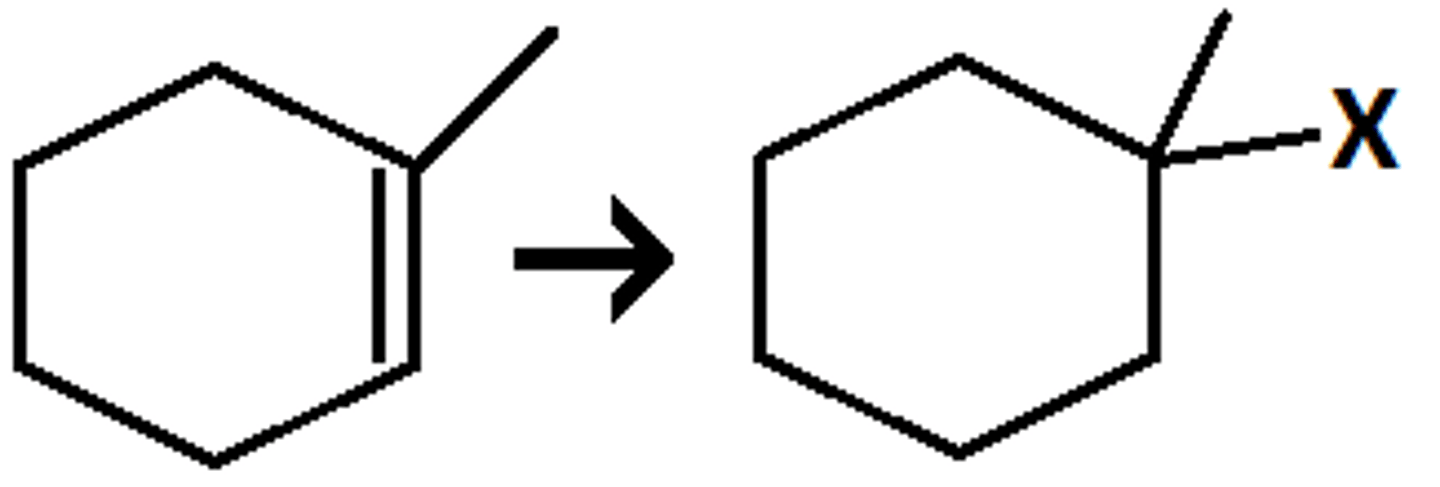
HX
(hydrohalogenation) markovnikov addition of H and X
- racemic mixture of enantiomers
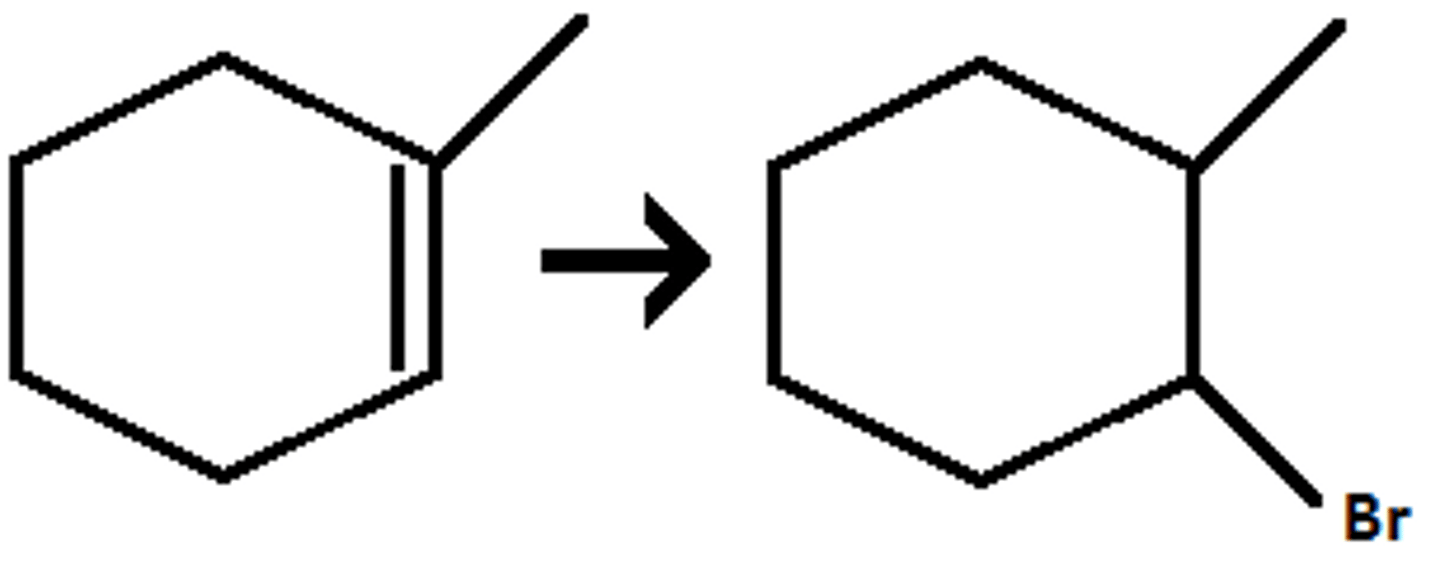
HBr / ROOR
(hydrohalogenation) anti-markovnikov addition of H and Br
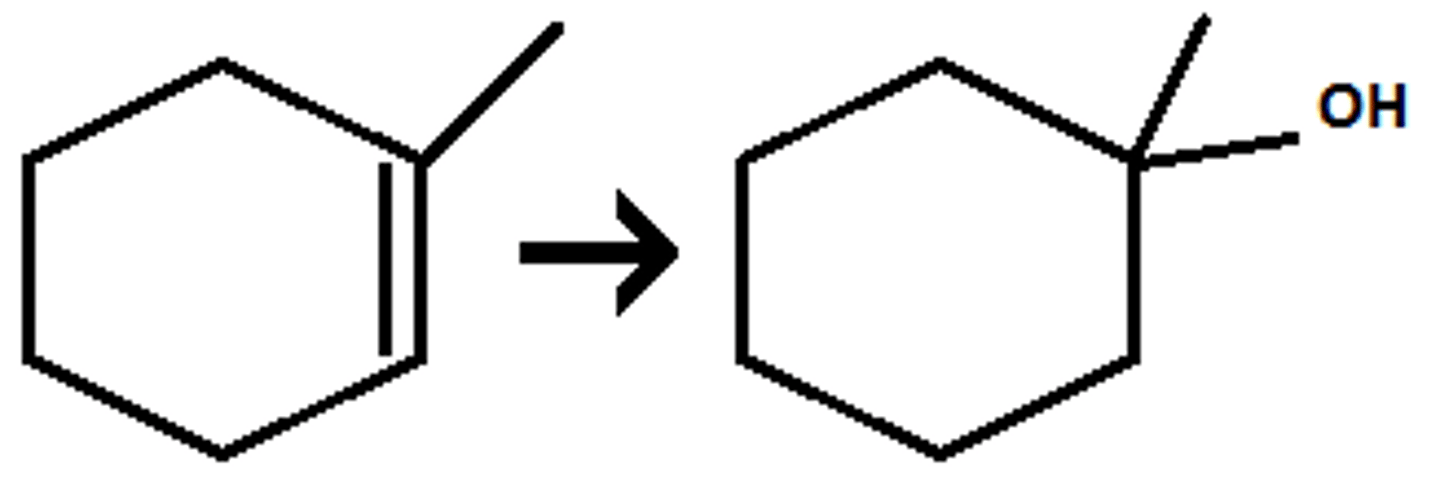
H₃O⁺
or
1)Hg(OAc)₂ / H₂O
2)NaBH₄
(acid catalyzed hydration/oxymercuration-demercuration) markovnikov addition of OH
- in dilute acid addition is favored
- in concentrated acid elimination is favored
- in 1)Hg(OAc)₂ / H₂O 2)NaBH₄, high yield is obtained
1) BH₃∙THF
2) H₂O₂ / NaOH
(hydroboration-oxidation) anti-markovnikov addition of OH
- syn addition (NOT anti position); only two of the four enantiomers are formed
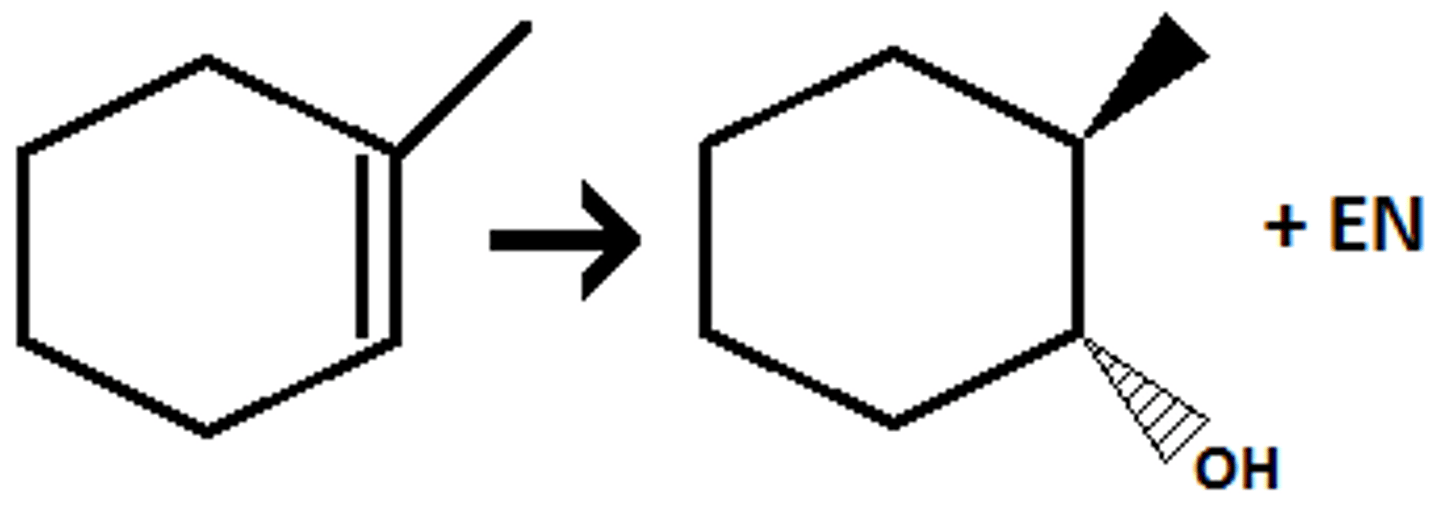
H₂ / Pt
(hydrogenation) syn addition of two H's
- syn addition (NOT anti position); only two of the four enantiomers are formed
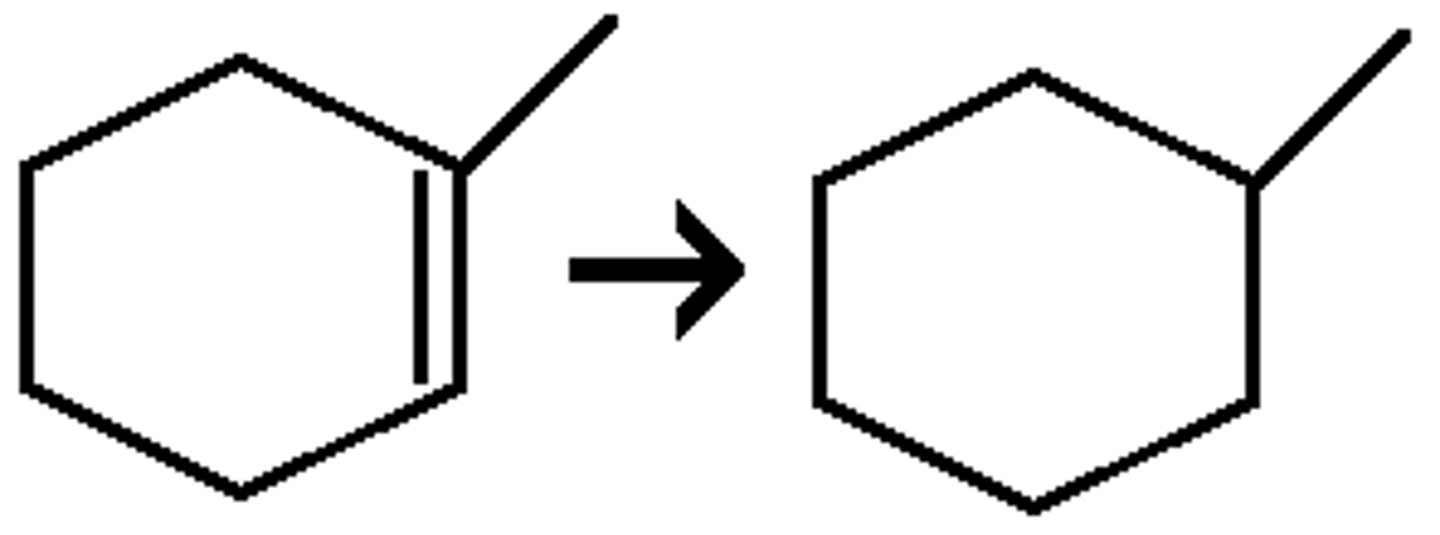
Br₂ / CHCl₃
(bromination) anti addition of two Br's (it can also be another halogen like I or Cl)
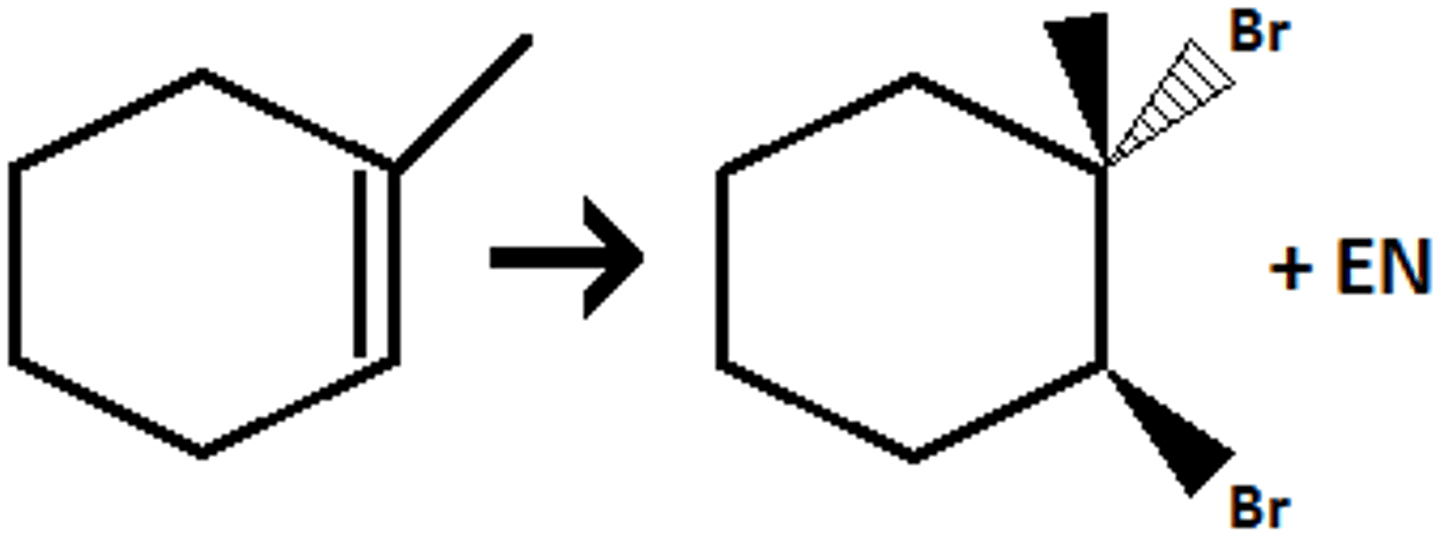
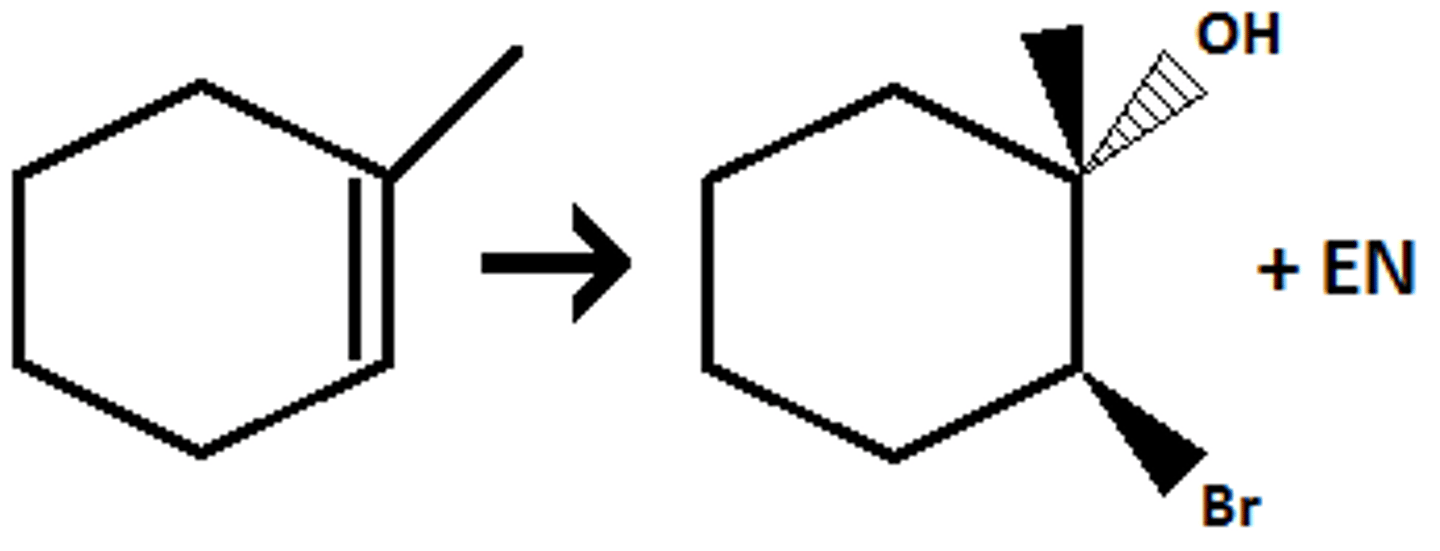
Br₂ / H₂O (HOR to add OR group)
(halohydrin formation) anti addition of Br (or Cl or I) and OH (OH goes to more substituted position)
- H₂O --> Br₂ and OH
- CH₃OH --> Br and OCH₃
- C₂H₅OH --> Br and OC₂H₅
- CH₃SH --> Br and SCH₃
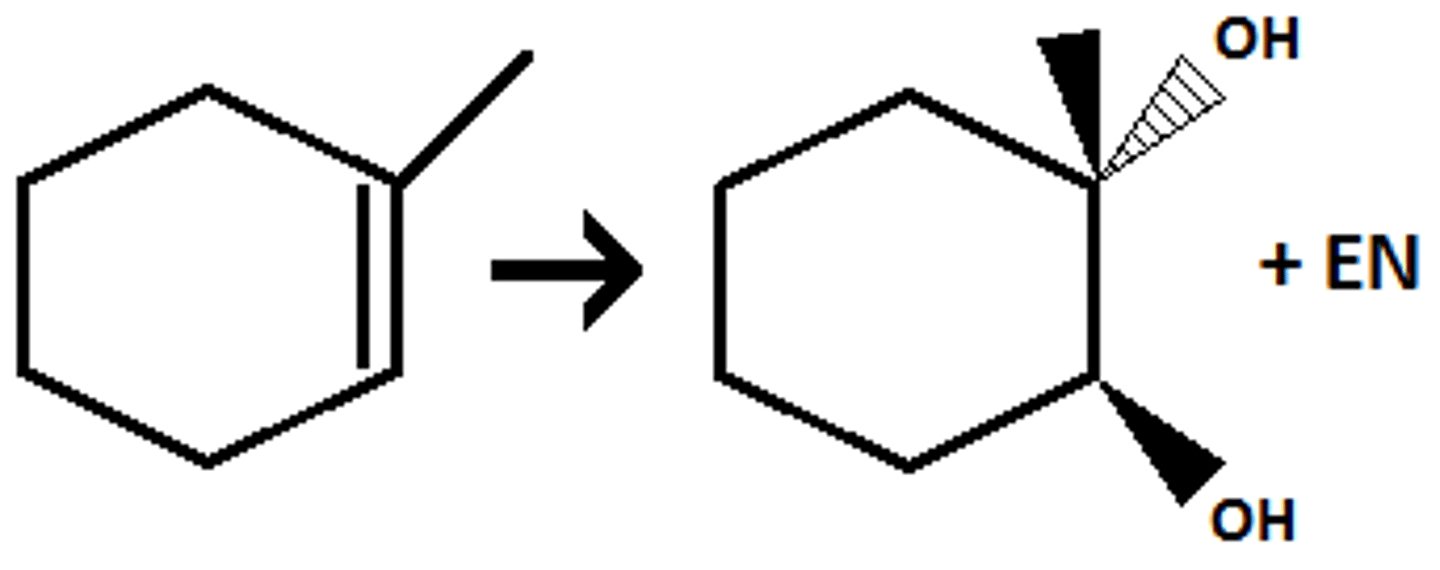
1) RCO₃H or MCPBA
2) H₃O⁺
(anti dihydroxylation) anti addition of two OH's
1) OsO₄
2) NaHSO₃ / H₂O
or
KMnO₄ / NaOH
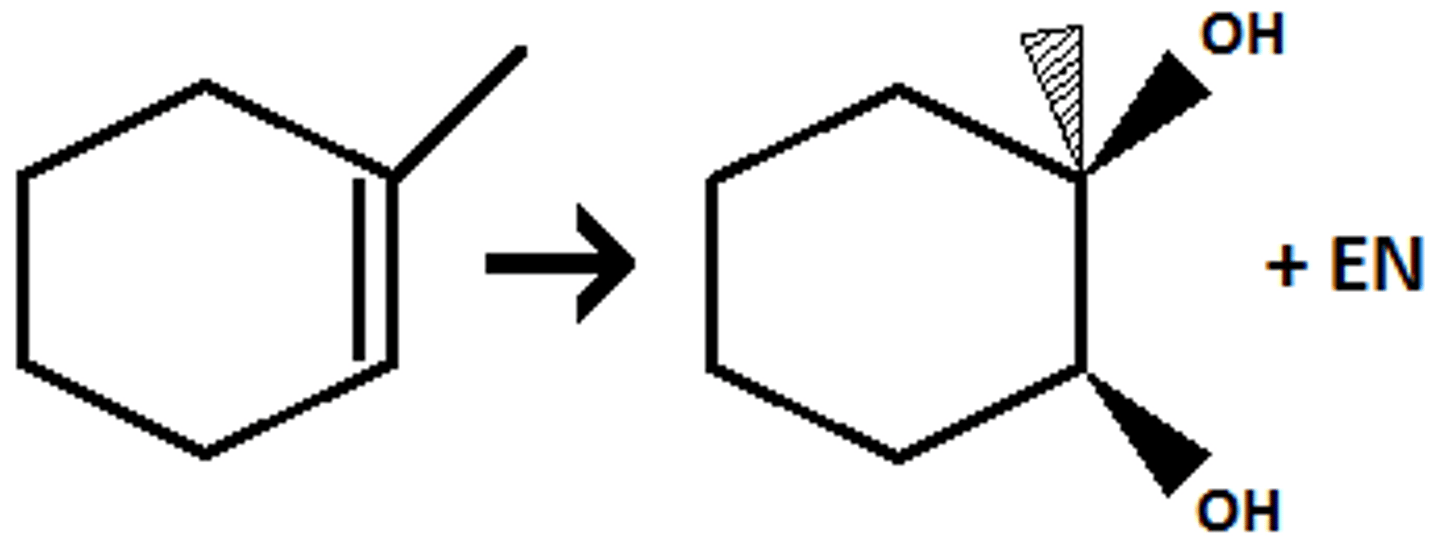
(syn dihydroxylation) syn addition of two OH's
- OsO₄ gives very high yield product but its toxic
- KMnO₄ gives medium yields but is non-toxic
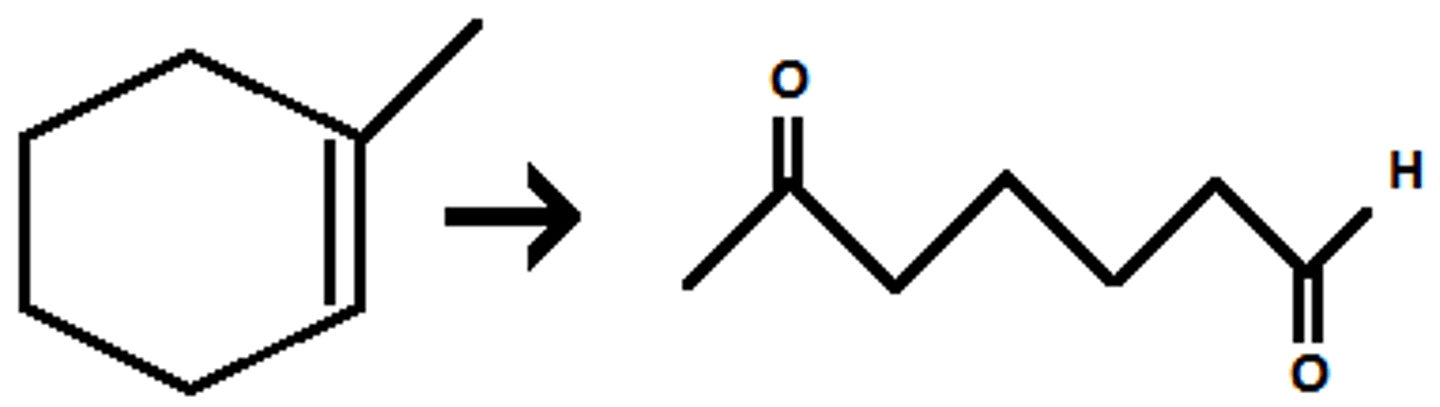
1) O₃
2) DMS
(ozonolysis/oxidative cleavage) breaks all double bonds and binds O to previously double bonded carbons
markovnikov rule
the h atoms in the addition reaction go to the c with the most substituents
anti-markovnikov rule
the h atoms in the addition reaction go to the c with the least substituents
high T
favors elimination
low T
favors addition
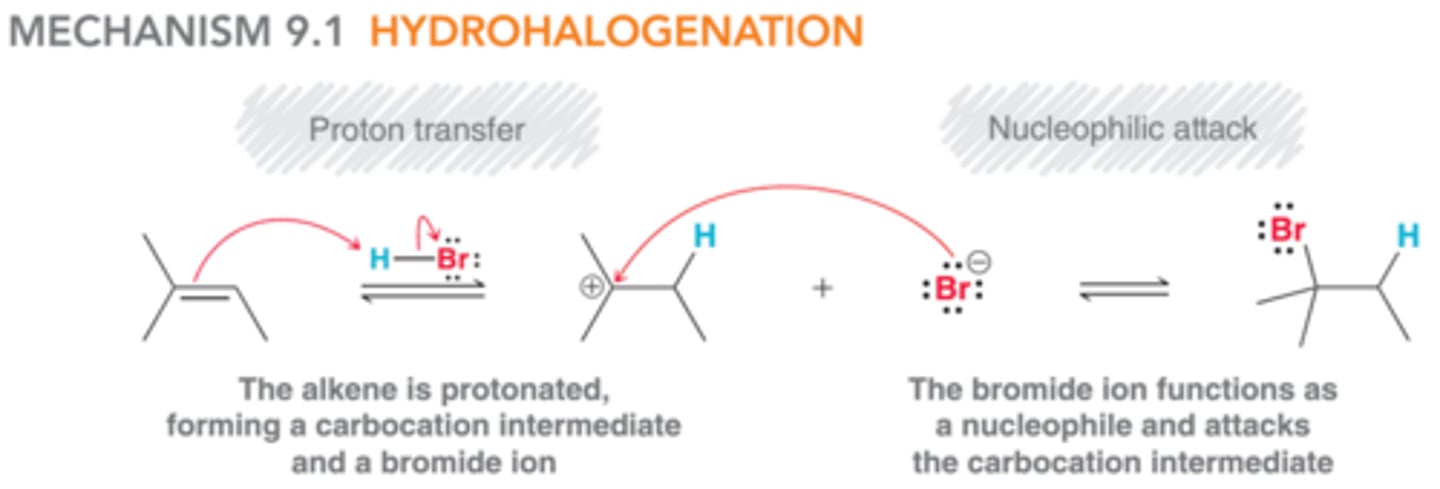
mechanism: hydrohalogenation
HX
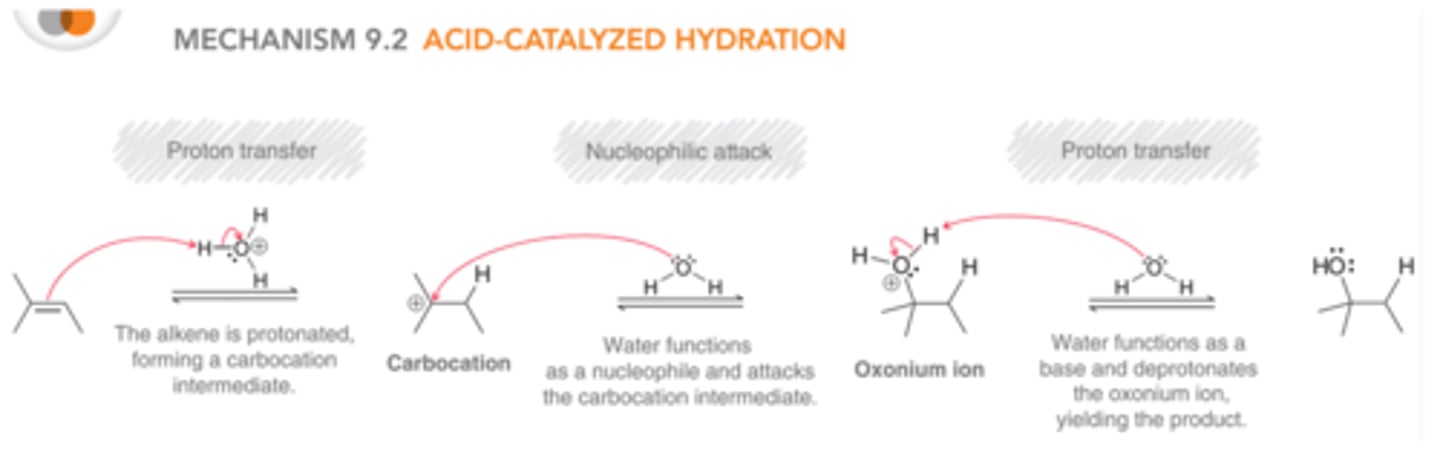
mechanism: acid-catalyzed hydration
H₃O⁺
1) Hg(OAc)₂, H₂O
2) NaBH₄
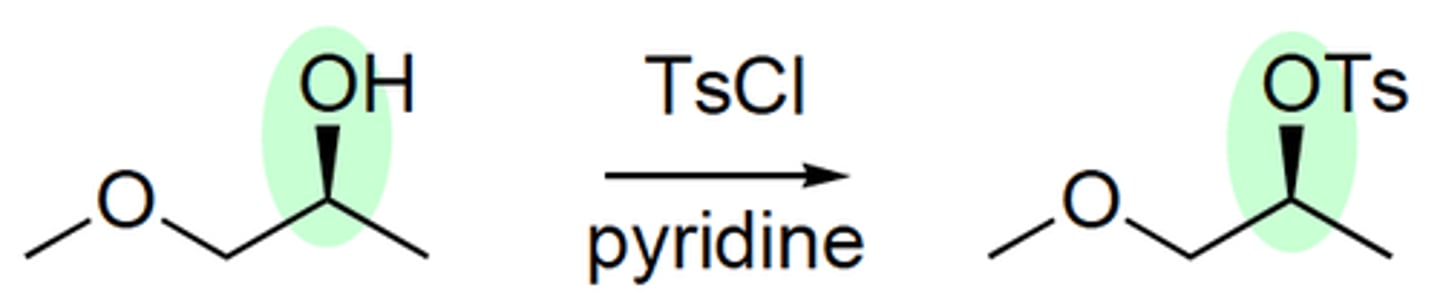
TSCL / Pyridine
changes OH group to OTs which is a better leaving group
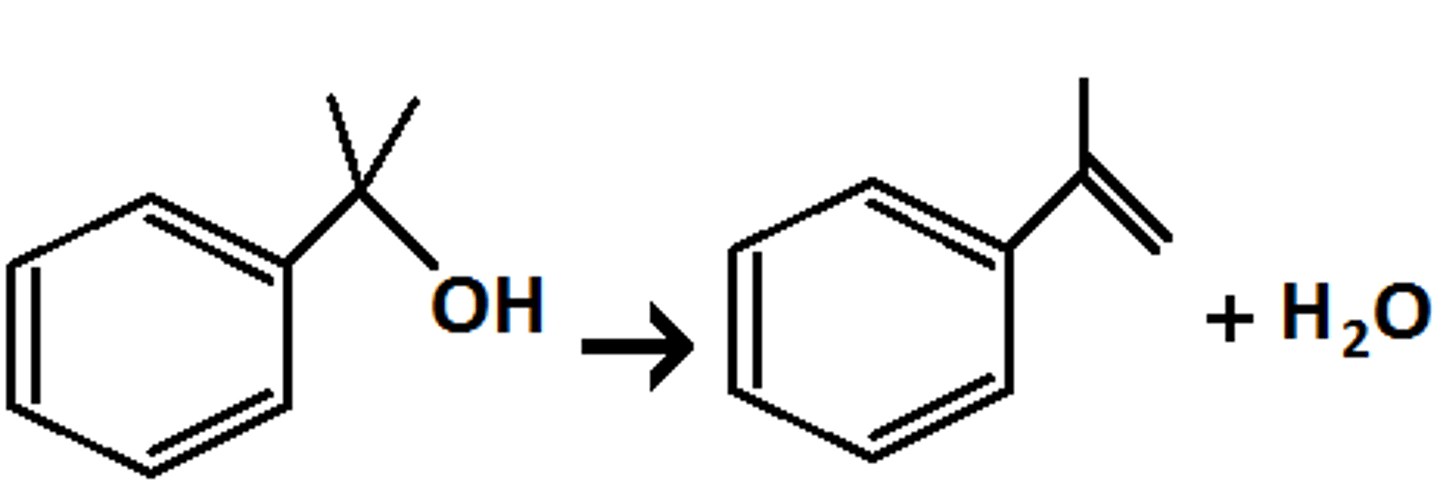
concentrated H₂SO₄
easily and efficiently eliminates OH group
- acts like a small base
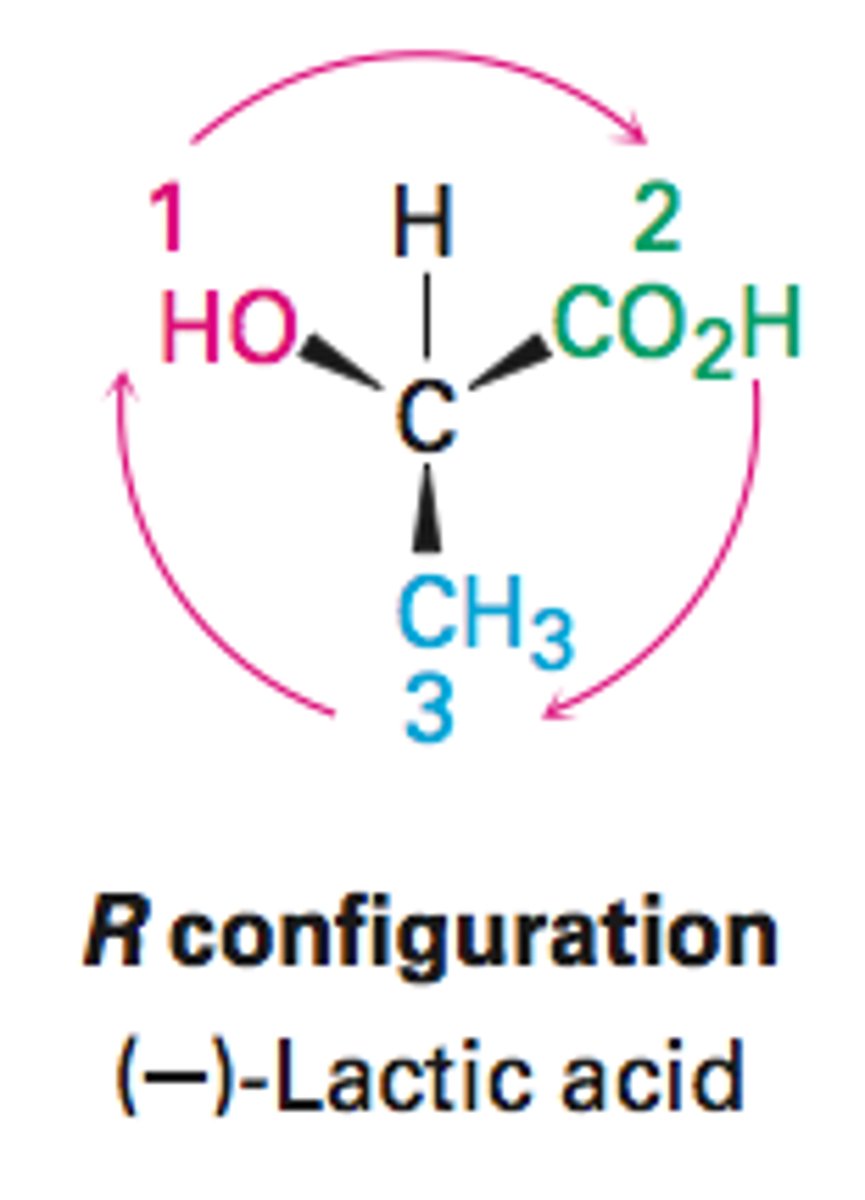
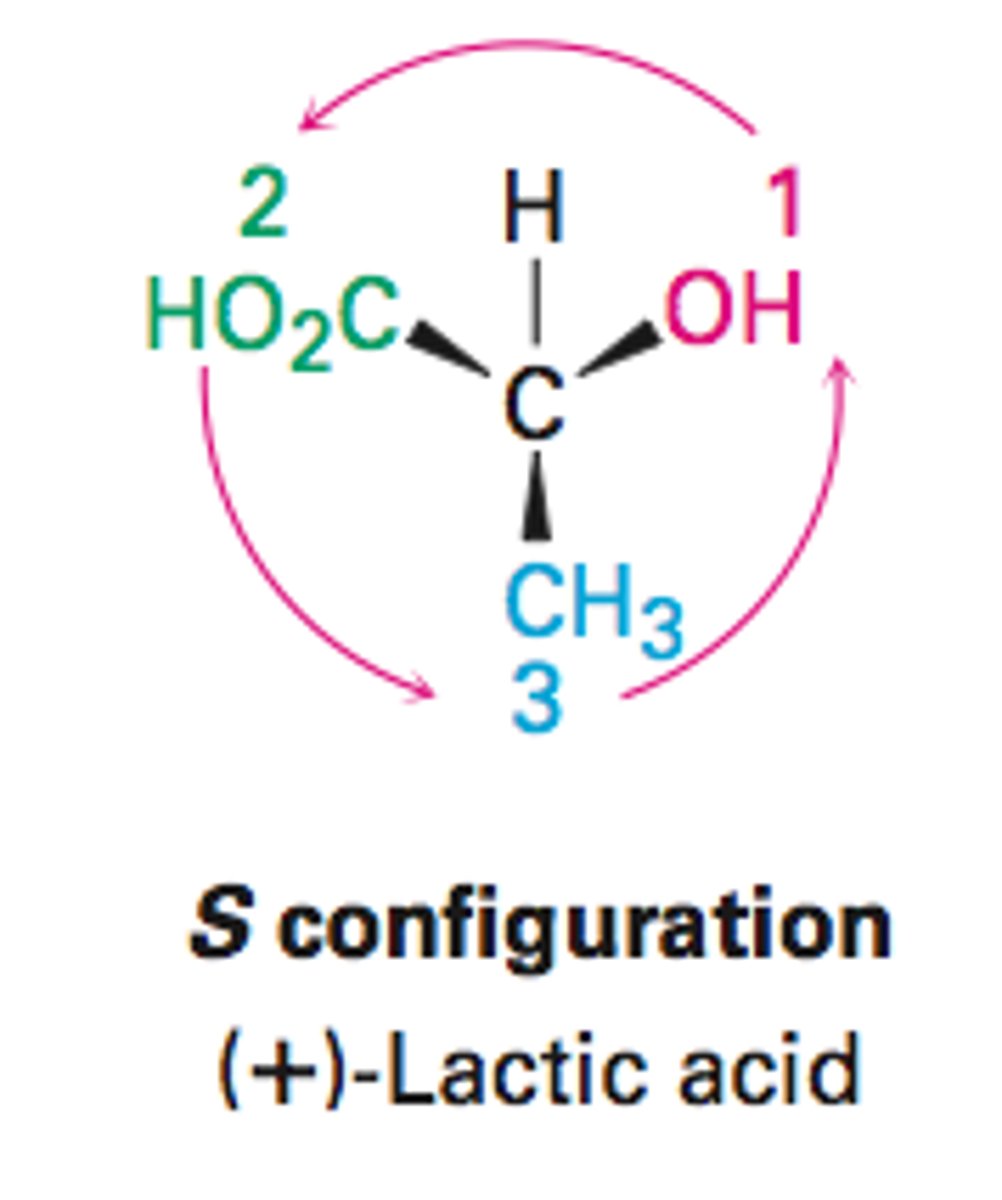
r configuration
clockwise
s configuration
counterclockwise
tert butyl
isopropyl