WJEC AS Biology Unit 1.1 - Chemical Elements are Joined Together to Form Biological Compunds
1/241
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
242 Terms
The key elements present as inorganic ions in living organisms
Magnesium (Mg2+)
Iron (Fe2+)
Calcium (Ca2+)
Phosphate (PO43-)
Magnesium (Mg2+) ion function
Constituent of chlorophyll and is therefore essential for photosynthesis. Plants without Mg in their soil cannot make chlorophyll and so the leaves are yellow, a condition known as chlorosis.
Mammals need magnesium for their bones. Bound to calcium and phosphorus in the skeleton
Magnesium (Mg2+) ion location
Constituent of chlorophyll
In soil
Bones in mammals
Iron (Fe2+) ion function
Constituent of haemoglobin in the blood of animals, which transports oxygen in red blood cells.
In plants = for growth and development
Iron (Fe2+) ion location
In animals = haemoglobin
Calcium (Ca2+) ion function
strengthening tissues
In mammals; bones and teeth
In plants; main components in structure, rigidity and strength of plant cell walls
Contributes to release of hormones and neuro-transmitters
Calcium (Ca2+) ion location
Structural component of bones and teeth in mammals and is a component of plant cell walls,
Structural component of bones and teeth in mammals
Component plant cell walls, providing strength
Phosphate (PO43-) ion function
Making biological molecules; nucleic acids such as DNA and RNA, nucleotides such as ATP, constituent of phospholipids in biological membranes.
Phosphate (PO43-) ion location
nucleic acids, e.g. DNA/RNA
nucleotides, e.g. ATP (energy carrier)
Phospholipids (main component of plasma membranes)
Organic molecules
Molecules that have a high proportion of carbon atoms
Excludes simple oxides of carbon, e.g. CO2
Includes carbohydrates, proteins, lipids and nucleic acids
Inorganic molecules
Molecules/ions that have no more than one carbon atom
Includes simple oxides of carbon, e.g. CO2
Water - Polarity
Polar molecule (separated charges)
Dipole (positively charged end (hydrogen) and a negatively charged end (oxygen) but no overall charge
Water - Ability for form hydrogen bonds
When two water molecules are in close contact the opposing charges attract each other, forming a hydrogen bond.
Form between the positively charge on the hydrogen atom of one molecule and the negative charge on an oxygen atom of another.
Individually, the hydrogen bonds are weaker, but together, the large number of them present in water form a lattice framework = strong + difficult to seperate —> wide range of physical properties vital to life
Water - Surface tension
High surface tension at ordinary temperatures
Cohesion between the water molecules at the surface produces surface tension so that, e.g. the body of an insect, such as a pond skater, is supported
Water - Solvent
Water molecules = dipoles
Positive and negative charged components of the molecule attract other charged particles, such as ions, and other polar molecules, such as glucose.
The ions and polar molecules can then dissolve in water, so chemical reactions take place in solution
Acts as a transport medium, e.g. plasma in blood, xylem/phloem.
Non-polar molecules, such as lipids, do not dissolve in water
Water - Specific heat capacity
High specific heat capacity
Large amount of heat energy is needed to raise its temperature
Hydrogen bonds between the water molecules restrict their movement, resisting an increase in kinetic energy —> resisting an increase in temperature
Prevents large fluctuations in water temperature (keeps aquatic habitats stable, organisms therefore don’t have to adapt to the extremes of temperature)
Allows enzymes within cells to work efficiently
Water - Latent heat of vaporisation
High latent heat of vaporisation
A lot of heat energy is required to change water from liquid to vapour
Process of evaporation transfers heat energy and is an effective way of cooling the body through sweating, panting, etc.
Important in temperature control, where heat is used to vaporise water from sweat on the skin or from a leaf’s surface
At the water evaporates, the body/surface cools
Water - Metabolite
Used in many biochemical reactions as a reactant
In hydrolysis and photosynthesis as a reactant
In condensation reactions and aerobic respiration, where water is a product
Water - Transport medium
Acts as a transport medium
Animals = in blood, where plasma transports dissolved substances
Plants = minerals dissolved in water are transported from root to leaves via xylem, and sucrose and amino acids in the phloem
Water - Chemical reactions
Solvent and metabolite
Transport of ions and polar molecules allows chemical reactions to take place when particles or molecules meet
Water - Cohesion
Attraction of water molecules for each other, seen as hydrogen bonds, resulting from the dipole structure of the water molecule
Individually, hydrogen bonds = weak, but because there are many of them, the molecules stick together in a lattice. This sticking together is called cohesion
Allowed columns of water to be drawn up xylem vessels in plants
Water - Transparency
Transparent, allowing light to pass through
Lets aquatic organisms to photosynthesise effectively
Water - Density
Denser than air
Provides a habitat support and buoyancy
Ice is less dense than water as hydrogen bonds hold the molecules further apart than they are in the liquid
Ice = good insulator, prevents heat loss
Dipole
A polar molecule, with a positive and a negative charge, separated by a very small distance
Hydrogen bond
The weak attractive force between the partial positive charge of a hydrogen atom of one molecule and a partial negative charge on another atom, usually oxygen or nitrogen
Carbohydrates
organic compounds which contain the elements carbon, hydrogen and oxygen
The basic unit = a monosaccharide
Monosaccharides
Small organic molecules
General formula Cn(H2O)n
Names are determined by the number of carbon atoms in the molecule
Building blocks for the larger carbohydrates
The carbon atoms within them make a ring when the sugar is dissolved in water, and they can alter their binding to make straight chains, with the rings and chains in equilibrium
Monosaccharides - properties
Can easily dissolve in water —> sweet tasting solutions
Cannot be broken down into simpler sugars - single monomer sugars
Monosaccharides - function
source of energy in respiration (carbon-hydrogen and carbon-carbon bonds are broken to release energy, which is transferred to make ATP)
building blocks for larger molecules (e.g. glucose is used to make polysaccharides starch, glycogen and cellulose)
intermediates in reactions (e.g. trioses, are intermediates in the reactions of respiration and photosynthesis)
constituents of nucleotides (e.g. deoxyribose in DNA, ribose in RNA, ATP and ADP)
What happens when two monosaccharides join together
Condensation reaction
Join from one carbon to the other, water is formed as a bi product
Forms a disaccharide
Examples of Monosaccharides
Triose
Pentose
Hexose sugars
Glucose structure
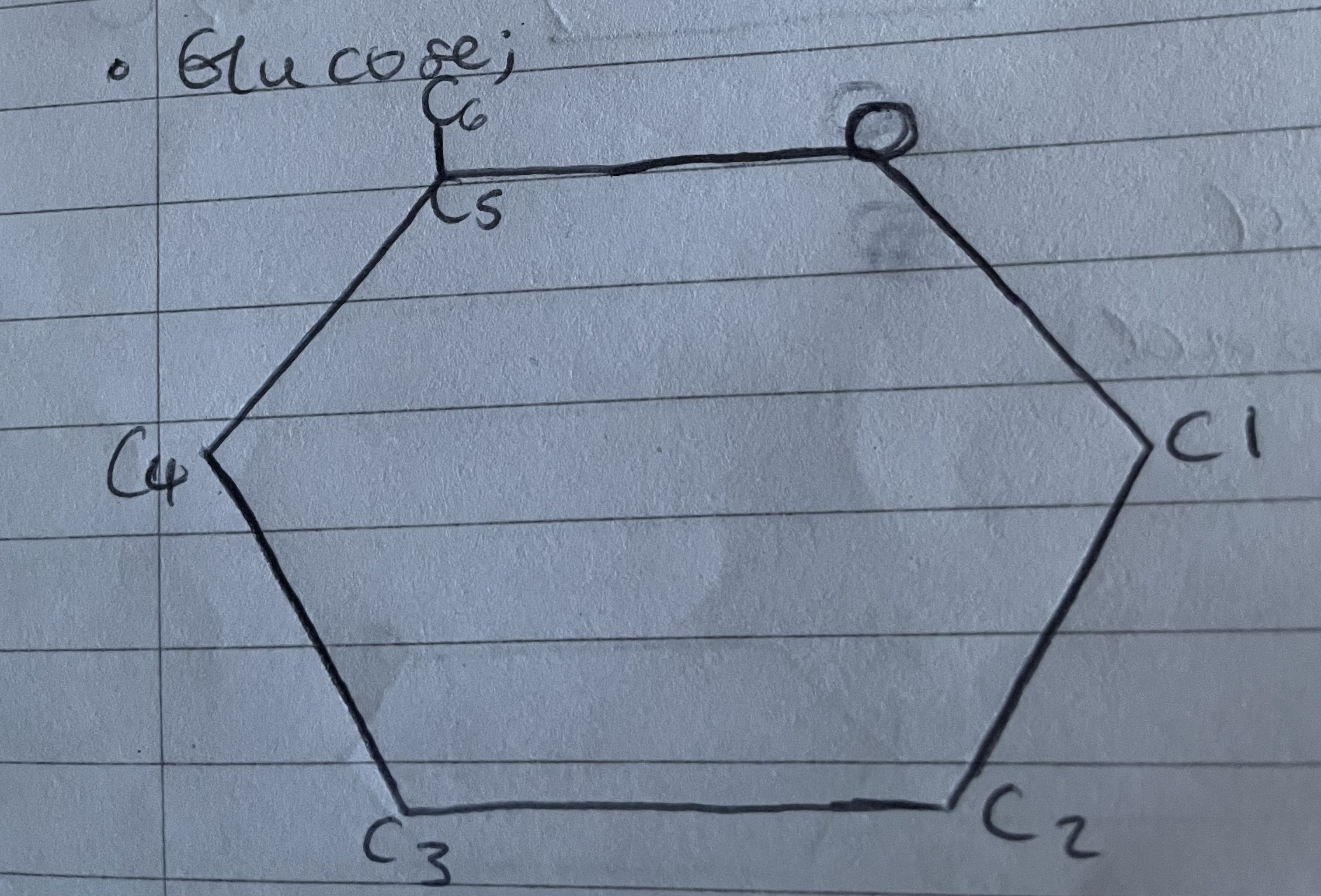
Glucose properties
Sweet in taste
Polar compound that readily dissolves in water
Reducing sugar that gives positive Benedict’s test
Cannot undergo hydrolysis
Glucose function
energy source
storage
synthesis (e.g. synthesis of carbohydrates)
regulation of blood sugar levels
organ function
Triose structure
C3H6O3
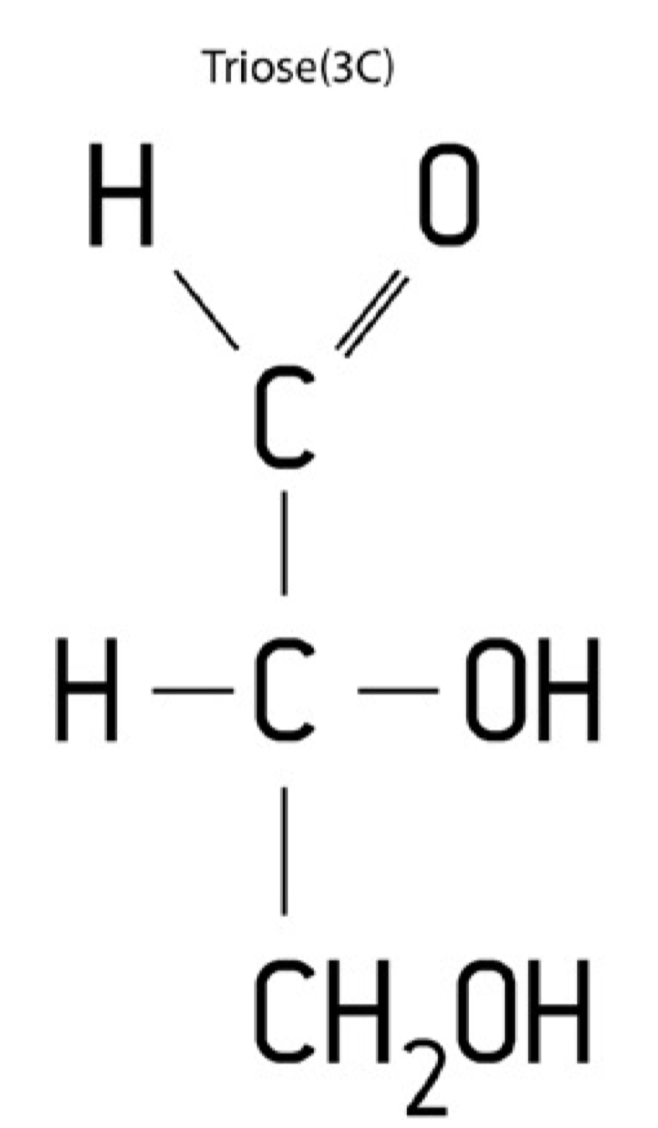
Triose properties
Monosaccharide containing three carbon atoms
Example; (glyceraldehyde)
Triose function
important in metabolism. Triose sugars are intermediates in the reactions of respiration and photosynthesis
Pentose structure
C5H10O5
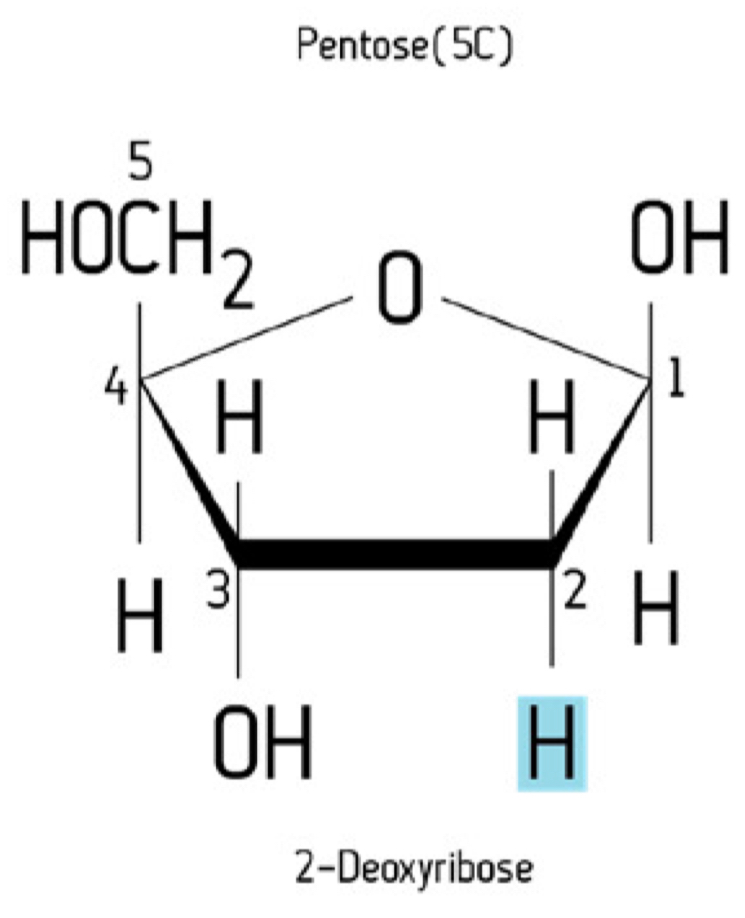
Pentose properties
Monosaccharide containing 5 carbon atoms
Examples; ribose and deoxyribose
Pentose function
Constituents of nucleotides, e.g. deoxyribose in DNA, ribose in RNA, ATP and ADP
Hexose structure
C6H12O6
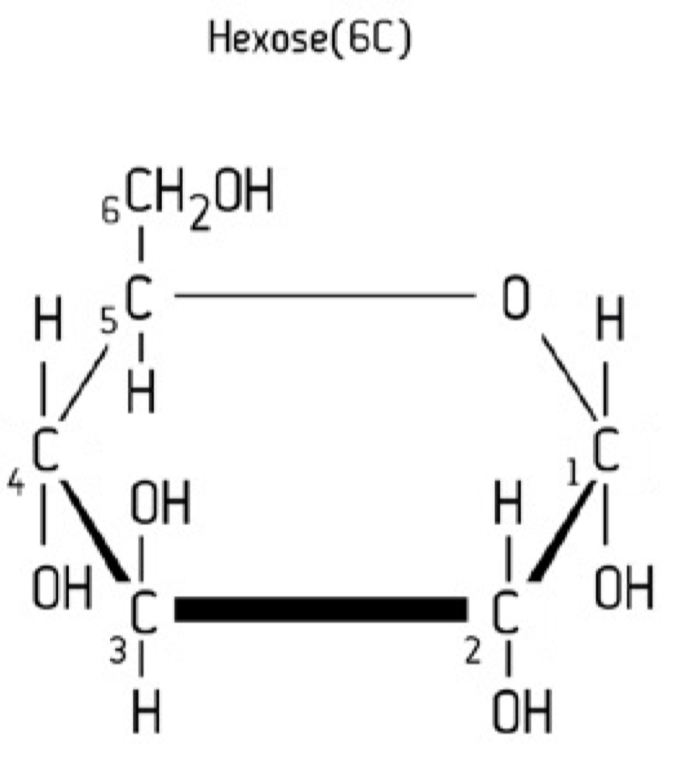
Hexose properties
Monosaccharide containing 6 carbon atoms
Examples; alpha-glucose, beta-glucose, fructose and galactose
Hexose function
Glucose is a hexose sugar
Glucose is a source of energy in respiration (carbon-hydrogen and carbon-carbon bonds are broken to release energy, which is transferred to make ATP)
Disaccharides
Composed of two monosaccharide units bonded together with the formation of a glycosidic bond and the elimination of water
This is an example of a condensation reaction
Disaccharide structure
General formula; C12H22O11
Linked by glycosidic bonds in the alpha or beta orientation
Disaccharide properties
Polar compounds
Readily soluble in water due to hydrogen bonding
Sweet in taste
Cannot diffuse through cellular membranes
Formed by condensation reactions, broken down by hydrolysis
Disaccharide function
Energy source
Body breaks them down into monosaccharides for absorption
Transport
Storage
Bodily function
Blood glucose regulation
Biological structure
Examples of Disaccharides
sucrose
lactose
maltose
Sucrose structure
Made up of a glucose and a fructose molecule joined together by a glycosidic bond
This glycosidic bond is formed between the carbon 1 of glucose and the carbon 2 of fructose. It is formed between the functional groups of the two molecules
The fructose molecule has a beta orientation while the glucose molecule has alpha orientation
Sucrose’s component disaccharide
glucose + fructose
Sucrose properties
Sweeter than glucose
Soluble in water
White crystalline solid in appearance
Non-reducing sugar
Sucrose function
A product of photosynthesis which is transported in phloem of flowering plants
Lactose structure
Made up of glucose and galactose molecules attached via a glycosidic bond
A C1-C4 glycosidic bond as it attaches the first carbon of glucose to the fourth of galactose
Both the glucose and galactose molecules have alpha orientation in lactose
Lactose’s component disaccharide
glucose + galactose
Lactose properties
Soluble in water, but its solubility is less than sucrose
Less sweet than sucrose
Lactose function
Found in mammalian milk
Maltose structure
Disaccharide made up of two subunits of glucose
Both glucose molecules are attach via a 1-4 glycosidic bond
This bond attaches the carbon 1 of one glucose molecule to the carbon 4 of the second glucose molecule
Both glucose molecules have alpha orientation
Maltose’s component disaccharide
Alpha- glucose + Alpha-glucose
Maltose properties
Reducing sugar
Soluble in water
Sweet in taste
Upon hydrolysis, it yields two glucose molecules
Maltose function
In germinating seeds
Polysaccharides
Large, complex polymers
Formed from very large numbers of monosaccharide units, which are their monomers, linked by glycosidic bonds formed by condensation reactions
Examples of Polysaccharides
starch
glycogen
cellulose
chitin
Starch properties
Compact structure of helical amylose and branched amylopectin make it energy dense
Insoluble in water
Additional units of glucose can be easily added to numerous ends or removed by hydrolysis
Not osmotically active as insoluble in water + chemically unreactive
Starch function
storage of glucose units + therefore storage of energy in plant cells. Can be stored in tubers like potatoes
Broken down releasing glucose units. The glucose units are used in respiration to produce ATP
Starch structure
Made of two polysaccharide components; amylose and amylopectin
Amylose; Polymer of alpha-glucose linked by alpha-1, 4-glycosidic bonds. Molecules can be thousands of residues/units long. Coils into a helix. Unbranched structure.
Amylopectin; Polymer of alpha-glucose monomers with a branched structure. Units of glucose within a chain are joined by alpha-1, 4-glycosidic bonds, branches formed by alpha-1, 6-glycosidic bonds, every 25-30 residues. Molecules can be up to million units of glucose.
The branched amylopectin and helical amylose form a compact structure.
Starch’s adaptations for function
Compact structure —> energy dense and an ideal to store lots of glucose units, and therefore energy, in a small volume
Glucose = an important respiratory substrate
Molecules of glucose can be easily added, allowing quick storage, or hydrolysed from the ends releasing glucose quickly for respiration.
Insoluble in water so does not affect the osmotic balance of cells.
Glycogen properties
Compact structure and high energy density
Many branches create many ends meaning many glucose units can be added (by condensation reactions) and units removed quickly (by hydrolysis reactions)
Insoluble in water/low solubility in water
Glycogen function
Glucose store in humans and therefore energy store. Particularly found in the liver and the muscles.
Plays an important role in regulating blood sugar levels in the blood; excess glucose can be added to the numerous ends of glycogen, reducing blood sugar quickly or removed from numerous ends to release glucose into the blood, raising blood sugar levels quickly.
Glycogen structure
Polymer of alpha-glucose
The units of glucose are within chains. Units are connected by alpha-1, 4-glycosidic bonds.
Has many branches
Branches formed by alpha-1, 6-glycosidic bonds
The branches are every 8 to 12 units
Glycogen’s adaptations for function
Compact structure + high energy density —> ideal energy store as a lot of energy can be stored in a small volume
Storage of glucose as glycogen in the liver plays an important role in regulating blood sugar levels. Many branches create ends, so lots of glucose units can be added quickly and removed quickly, allowed blood sugar levels to be reduced quickly or raised quickly
Low solubility means it is cosmetically inert and therefore does not affect osmosis in cells
Cellulose properties
High tensile strength
Rigid
Insoluble in water, soluble in organic solvents
Cellulose fibres are freely permeable, because there are spaces between the fibres. Water and its solutes can penetrate through these spaces in the cell walls, to the cell membrane
Cellulose function
Major component of cell walls. Cell walls are rigid which maintains the shape and supports the cells
Energy source for animals
Cellulose structure
Polymer of beta-glucose
Units joined by beta-1, 4-glycosidic bonds
The beta-linkage rotates the adjacent monomers 180 degrees to each other, resulting in long and straight chains
Hydrogen bonds form between parallel chains
Chains are highly cross linked
Many chains are linked via hydrogen bonding to form a microfibrils
The microfibrils come together to form stronger fibres
Fibres form layers (laminar structure).
Layers arranged at different angles to each other, providing additional rigidity
Calcium is also involved in forming cross bridges, providing strength
Cellulose adaptations for function
Rigid and so when the cell contents push against the cell wall, turgor pressure is generated
This supports the shape of the cell wall and makes them form (turgid). Without it they become floppy/flaccid
Chitin properties
High tensil strength
Rigidity
Waterproof
Chitin function
In insects, it forms the exoskeleton
In fungi, chitin is found in the cell wall
Chitin structure
Made of beta-glucose units, joined by beta-1, 4-glycosidic bonds
Beta glycosidic bonds cause the adjacent monomers to be twisted 180 degrees to each other, forming straight chains
Hydrogen bonds age able to form between the chains —> makes chitin strong and gives it high tensile strength and rigidity
Some -OH groups of the glucose units are replaced by nitrogen-containing acetylamine groups
These groups contribute towards the hydrogen bonding between the chains, adding to its tensile strength
These form microfibrils and fibres, with the fibres being arranged in groups at opposing angles, all adding to its rigidity and strength
Chitin’s adaptations for function
Rigid + therefore provides a tough, rigid exoskeleton that gives shape and supports the insect’s body
Insects tend to live in dry/arid habitats; because the chitin is water proof, it reduces loss of water by evaporation, preventing desiccation, allowing the insect to survive dry conditions
In fungi, it is found in the cell wall; because it is rigid, it generates turf or pressure when the cell contents push against the cell wall. This gives the fungal cells shape and support. It also prevents too much water entering and resulting in osmotic lysis of the cell
Structural isomer
Molecules that have the same molecular formula but different structural formulae
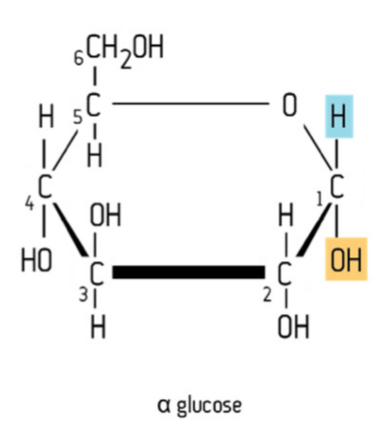
Alpha structural isomerism in glucose
AT CARBON 1;
-OH below
-H above
This therefore affects the bond between the unit sugars in disaccharides and polysaccharides. Same chemical formula as beta-glucose; C6H12O6
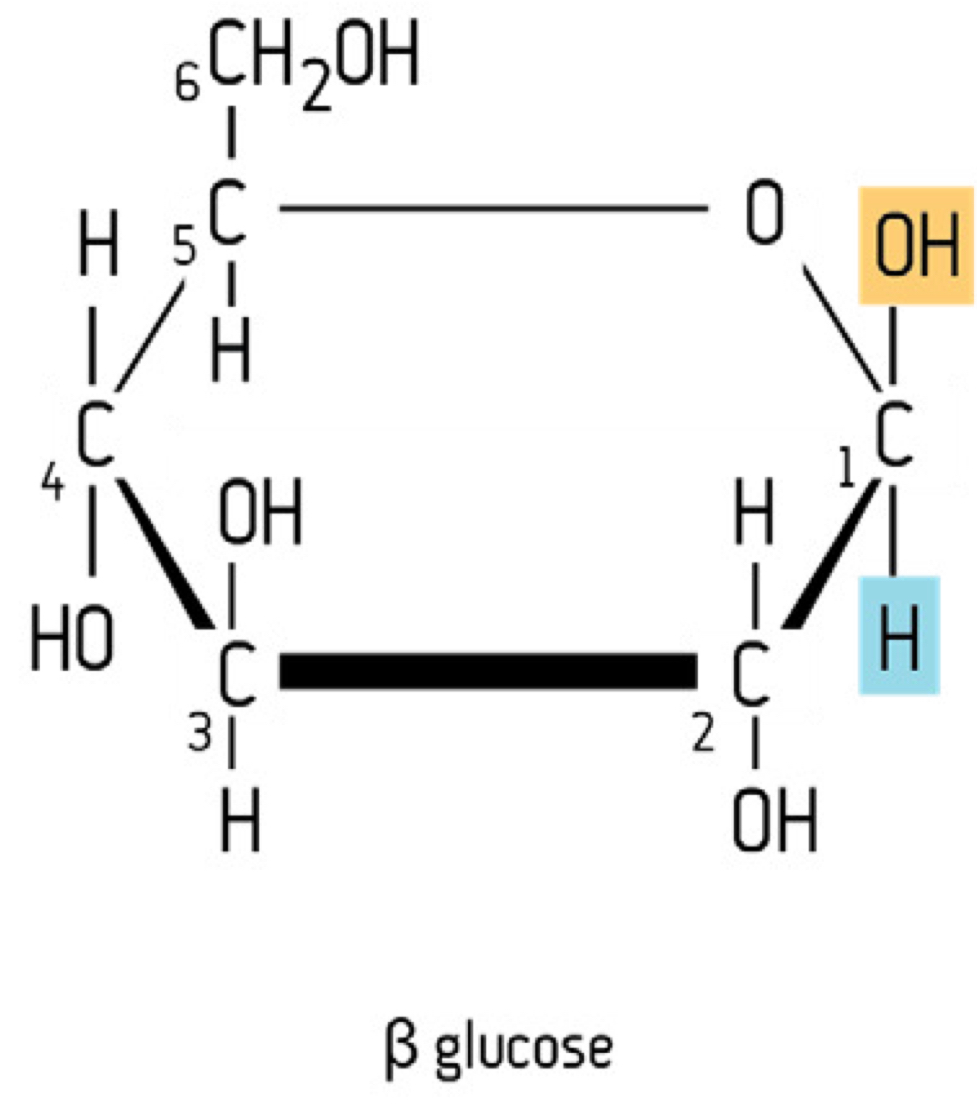
Beta structural isomerism in glucose
AT CARBON 1;
-OH above
-H below
This therefore affects the bond between the unit sugars in disaccharides and polysaccharides. Same chemical formula as alpha-glucose; C6H12O6
Polymers
A large molecule comprising repeated units, monomers, bonded together
Monomers
Single repeating units of a polymer
Polymerisation of glucose into storage and structural carbohydrates
Formed from very large numbers of monosaccharide units, which are their monomers, linked by glycosidic bonds
Polymerisation of glucose into starch
Several simple and soluble molecules of glucose age put together to form a complex, insoluble molecule of starch
Polymerisation of glucose into glycogen
Glycogenesis
Several simple and soluble molecules of glucose are put together to form a complex, insoluble molecule of glycogen
Polymerisation of glucose into cellulose
several simple and soluble molecules of glucose are put together to form a complex, insoluble molecule of cellulose
Polymerisation of glucose into chitin
Several simple and soluble molecules of glucose are put together to form a complex, insoluble molecule of chitin
Lipids
Contain Carbon, Hydrogen and Oxygen
In proportion to carbon and hydrogen, contain much less oxygen
Non-polar compounds —> insoluble in water, soluble in organic solvents
Lipid structure
Most lipids are triglycerides which are made of glycerol and three fatty acids
Others are phospholipids which are made of a hydrophilic phosphate group, glycerol and two fatty acids
Lipid function
Good source of energy (high yield per gram)
Phospholipids; biological membranes and electrical insulation
Triglycerides; energy reserves in plants and animals as lipids contain more carbon-hydrogen bonds than carbohydrates, thermal insulation, protection of organs, produce metabolic water when oxidised
Waxes; reduce water loss
Lipid properties
Differences in properties come from variations in the fatty acids
Can exist as fats or oils
Insoluble in water because they are non-polar
Store a lot of energy
Poor conductors of heat
Lipid adaptations for function
High yield of energy per gram (when oxidised/used in respiration) due to compact structure
Insoluble in water so does not affect osmosis
Triglycerides
Formed by the combination of one glycerol molecule and three molecules of fatty acids
Glycerol molecule is always the same but the fatty acid component varies
The fatty acids join to glycerol by condensation reactions, where by three molecules of water are removed and ester bonds are formed between the glycerol and fatty acids, forming a triglyceride
Triglyceride structure
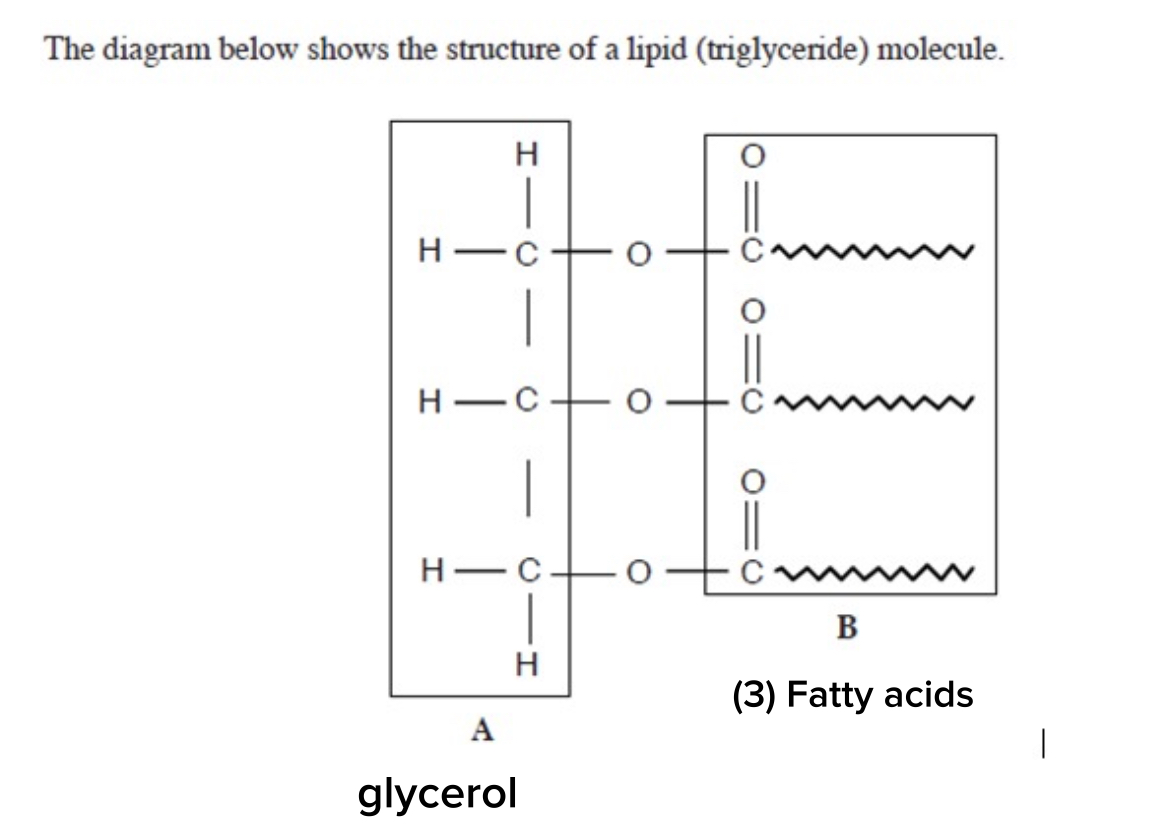
Triglyceride function
Insulation
Energy store/source
Protection
Triglyceride properties
Non-polar —> insoluble in water —> don’t affect osmosis within cells
Triglyceride location
Body fat under skin/around organs
Triglyceride adaptations for function
High yield of energy per gram (when oxidised/used in respiration) due to compact structure
Insoluble in water so does not affect osmosis
Phospholipids
One end of each molecule is soluble in water
Consists of hydrophilic phosphate group, glycerol and two hydrophobic fatty acid tails