Rate of reaction
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What is rate of reaction?
The speed at which the products are formed from the reactants in a chemical reaction.
What is a catalyst?
A substance that increases the rate of a chemical reaction without being used up
Factors affecting rate of reaction
There are four factors that affect the rate (speed) of a chemical reaction:
temperature
concentration
particle size
use of a catalyst
What is activation energy?
The minimum amount of energy needed to start a chemical reaction.
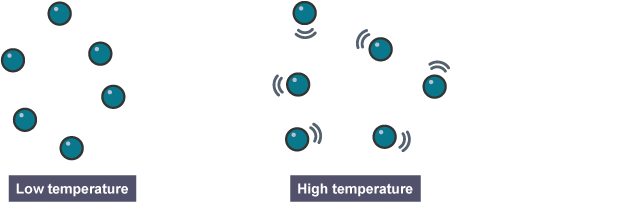
How does temperature affect rate of reaction?
If the temperature is increased, the particles have more energy and so move quicker.
Increasing the temperature increases the rate of reaction because the particles collide more often and with more energy.
The higher the temperature, the faster the rate of a reaction will be.
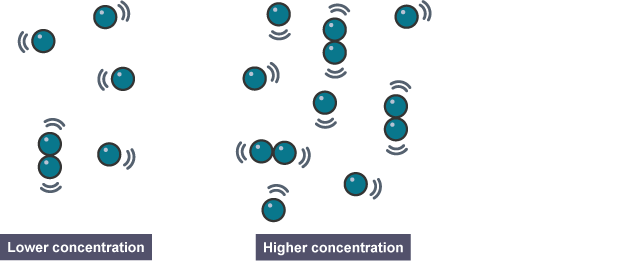
How does concentration affect the rate of reaction?
If the concentration of reactants is increased, there are more reactant particles moving together.
There will be more collisions and so the reaction rate is increased.
The higher the concentration of reactants, the faster the rate of a reaction will be.
How does particle size affect the rate of reaction?
By decreasing the particle size of a reactant, we are increasing its surface area.
The smaller the particle size the faster the reaction.
The greater the surface area, the higher the chance of collisions, thus the faster the rate of reaction.
How does a catalyst affect rate of reaction?
Provides an alternative reaction pathway
Has a lower activation energy
Increases the frequency of more successful collisions, because more particles have greater energy than the activation energy
What is the equation that links time, mean rate and volume?
mean rate= volume/ time
mean rate= (cm³) / (s)
List 2 things that are needed in order to have a successful collisions:
more frequent collisions
collisions have to have enough energy
What is concentration?
The volume of particles in a given space (liquid).
What is the equation for rate of reaction?
Rate of reaction= mass / time
What is the equation used to measure the rate of reaction? (extension of question before)
mass of reactants lost / time