Chem302 Unit 1 - Physical And Chemical Equilibria
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Combustion Reactions
Exothermic, ∆H (-)
Hydrocarbon + Oxygen → Carbon Dioxide + Water
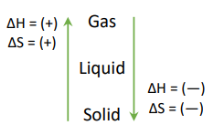
Entropy, ∆Ssys, is (+) when…
phase changes from solid→liquid→gas
num moles increases left to right
mixing or dissolving substances
molecule size or complexity generally increases
system at higher temp
system disorder (num microstates) increases
Phase Changes: ∆H and ∆S
∆H (-) for making IMFS, (+) for breaking IMFS
∆S freedom of movement
SIGNS FOR ∆G and ∆S NEED TO BE THE SAME FOR PHASE CHANGES
Temp Dependence of ∆G
Given ∆G = ∆H - T∆S
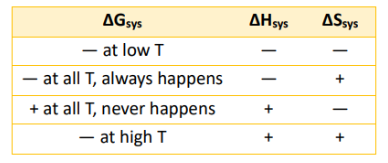
Transition Temp
Phase Transition Temperature from Gibb’s Free Energy Equation
Tphase = ∆H/∆S
Vapor Pressure Theory
VP surface phenomena
IMF inversely related to VP
VP independent of volume bc pressure is collisions with container
Clausius Clapeyron Equation and Theory
ln(P2/P1) = (∆Hvap/R)/(1/T1 - 1/T2)
as T increases, VP increases exponentially
∆Hvap is always positive
as IMF increases, ∆Hvap increases, causing VP to decrease exponentially
as IMF increases, the slope increases on the graph (line becomes steeper)
∆Hvap values for common compounds
H2O: 40 kJ/mol
HOCH2CH2OH: 60 kJ/mol
Hexane: 30 kJ/mol
He: 2 kJ/mol
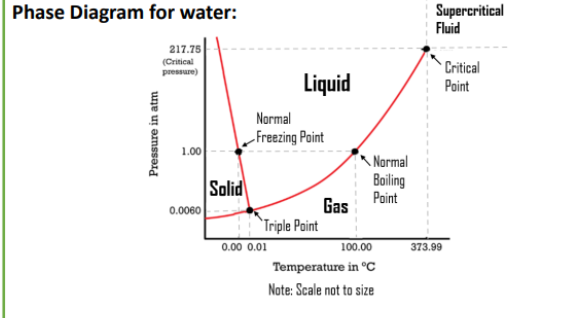
Phase Diagram Interpretation
lines denote the split between phases - crossing a line indicates a phase change
temperature on the horizontal axis, pressure on the vertical
critical point: set pressure and temperature above which the liquid and gas phases meld together to form a supercritical fluid
Phase Diagram for Water
WATER IS UNIQUE
negative slope for the solid-liquid line which indicates solid water is less dense than liquid water
Calculating ∆H across Phase Transitions
Solid Warming: q = mCsolid∆T
Solid Melting: q = m∆Hfus
Liquid Warming: q = mCliq∆T
Liquid Vaporizing: q = m∆Hvap
Gas Warming: q = mCgas∆T
Values to calculate Q across Phase Transitions for Water
Cice = 2 J/g°C
∆Hfus = 340 J/g
Cwater = 4 J/g°C
∆Hvap = 2260 J/g
Csteam = 2 J/g°C
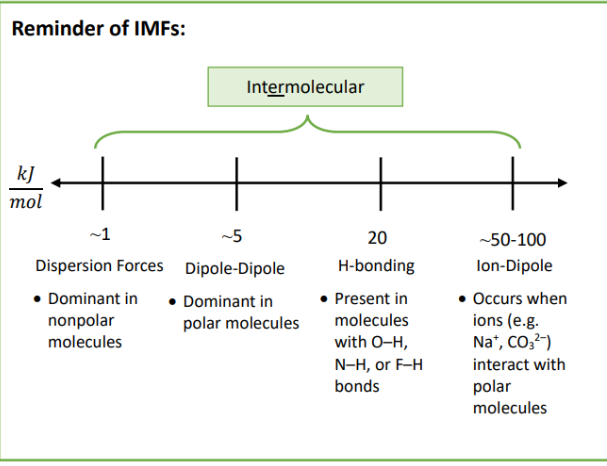
Polar Molecules
Permanent Dipoles
H-bonding
Asymmetrical
Typically polar functional groups (∆EN is large)
ie. -OH, -C-OH, -N-H
Non-Polar Molecules
Dispersion forces
Symmetrical
Hydrocarbons/Lots of alkyl (-CHx) groups
No permanent dipole or hydrogen bonding
ie. -CH2CH2CH3, SF6, O2
Miscibility
Means “mix with”
“Like dissolves like”
Non-polar mix with non-polar, but not with polar or with salts
Polar mix with polar and salts but not hydrocarbons
Molecules with polar and non-polar regions exist (origin of soaps)
Salt Dissociation in Water
∆Gsoln = ∆Hsoln - T∆Ssoln
if ∆Gsoln is (-), salt will dissolve
∆Hsoln can either be (+) [ie, NaCl] or (-) [ie, CaO]
∆Ssoln usually (+) bc dissolving = solid → aqueous, increasing microstates
∆Hsoln for Salt Dissociation
∆Hsoln = ∆HC.L. + ∆Hhyd
plug ∆Hsoln into ∆G equation
∆HC.L.defines energy needed to break bonds in crystal lattice (ALWAYS +)
∆Hhyd (∆Hsolvation) is amount of energy released upon forming solute-solvent IMFs (ALWAYS -)
determines at what temperature the salt dissociates at
Gas Interactions with Water
Reaction: compound has chemical rxn with water
KNOW THESE REACT: CO2 (H2CO3), NO2 (HNO3), SO3 (H2SO4)
Like Dissolves Like: Polar gases dissolve readily
Inert, non-polar gases:
∆Ssoln = (-) bc gas → liquid
∆Hsoln = (-) for gas—water bc IMF is forming
Since ∆S and ∆H both (-), ∆G (-) at low T
gas more likely to dissolve as temp decreases
Henry’s Law
As pressure of gas above a liquid solvent increase, the solubility of the gas in that solven tincreases proportionally.
Pgas increases as Solubilitygas increases
Vapor Pressure in a Binary System
Ptot = PA + PB
where Py = P°Xy
where P° is the pure vaor pressure of the compound
where Xy = (mol y)/(mol total) is the mole fraction
Common Applications of Vapor Pressure Lowering (Colligative Properties)
Resins and plasticizers can be used to reduce the evaporation rate of inks, films, and adhesives
Common Applications of Freezing Point Depression (Colligative Properties)
Salts are added to roads in the winter to thaw the ice
Salt is added when making ice cream to lower the freexing point
Antifreeze (ethylene glycol in water) prevents the colling system in cars from freexing in colder climates
Common Applications of Boiling Point Elevation (Colligative Properties)
Antifreeze ALSO increases boiling point, which mean that a radiator won’t boil on a really hot day.
Common Applications of Osmotic Pressure Differences (Colligative Properties)
Solvent in dilute solutions will move towards more concentrated solutions
Cells lyse (break open) when exposed to distilled (pure) water
Freshwater fish cannot survive in salt water
Calculation for Osmotic Pressure Change (Colligative Properties)
Π = iMRT
Π = osmotic pressure
i = van’t Hoff factor
M = molarity (mols solute)/(L solution)
T = temp in Kelvin
Calculation for Freezing Point Depression/Boiling Point Elevation (Colligative Properties)
∆Tf = i • kf • m OR ∆Tb = i • kb • m
∆Tf/b = change in freezing/boiling point from pure solvent
i = van’t Hoff factor
kf/b = freezing/boiling point constant
m = molality (mols solute)/(kg solvent)
van’t Hoff factor
essentially how many different pieces of a substance exist in solution
Covalent Compounds:
compounds won’t break when dumped into water, so i = 1
Ionic Compounds:
assuming soluble, will break when dumped into water, so i = num ions
ie. NaCl, Na+ and Cl-, so i = 2
Law of Mass Action
Reaction: aA + bB → cC + dD
Equilibrium Constant:
Kc = ([C]c[D]d) / ([A]a[B]b)
Kp = {(PC)c(PD)d} / {(PA)a(PB)b}
Exceptions:
gases can be pressure or concentration but not both
aqueous solutions of mixtures written as concentrations with [ ] symbols
pure liquids and solids ahve a constant concentration which is absorbed into K and NOT included when writing Q or K
Reaction Direction from Q and K
Q = [right]/[left]
if Q < K, rxn shifts right
if Q > K, rxn shifts left
RICE Table
Reaction → write out reaction
Initial Concentration → write the corresponding initial concentrations in the table; pure liquids and solids have no concentration; compounds for which values aren’t given are assumedto be 0
Change → write the change of the compounds in terms of one variable, x
Equilibrium → find equilibrium values in terms of x
insert the equilibrium values into K equation and solve
Le Chatelier’s Principle and Reaction Direction
A system at equilibrium can be stressed by changing temp, pressure or concentration applied to the system.
System can shift to make more on the right or make more on the left.
Direction determined by whichever way reduces stress
Le Chatelier’s Principle: Concentration Stress
aA + bB → cC + dD
add A or B → rxn shift right to ↓ compound
add C or D → rxn shift left to ↓ compound
remove A or B → rxn shift left to ↑ compound
remove C or D → rxn shift right to ↑ compound
Le Chatelier’s Principle: Pressure Stress
pressure only affects gases
↑ in pressure → sys shift to ↓ gas mols
↓ in pressure → sys shift to ↑ gas mols
if ∆n positive
P↑ or V↓ → shift left
P↓ or V↑ → shift right
if ∆n negative
P↑ or V↓ → shift right
P↓ or V↑ → shift left
if ∆n = 0 → no shift due to P/V changes
Le Chatelier’s Principle: Temperature Stress
can easily add/remove heat from sys.
shifts away from heat/cold depending on exothermic/endothermic
Generally:
endothermic: right if heat(+), left if heat(-)
exothermic: right if heat(-), left if heat(+)
van’t Hoff Equation
ln(K2/K1) = ∆Hrxn/R (1/T1 - 1/T2)
∆Hrxn sign inverts depending on which way you write the rxn
rxn exothermic, shift right when T is lower (K2 < K1)
rxn is endothermic, shift right when T is higher (K2 > K1)
Relationship of ∆G° to K
∆G° = -RTlnK
a large negative ∆G° results in rxn shifting far to the right to completion; K with a large positive exponent
a large positive ∆G° results in system staying on the left; K with a large negative exponent