Topic 7 - Energy changes
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
7.9 Recall that changes in heat energy accompany the following
changes:
a salts dissolving in water
b neutralisation reactions
c displacement reactions
d precipitation reactions
and that, when these reactions take place in solution,
temperature changes can be measured to reflect the heat
changes
● Salts dissolving in water is either exothermic or endothermic
● Neutralisation reaction is exothermic
● Displacement is an exothermic or endothermic reaction
● Precipitation is an exothermic reaction
● if these reactions take place in a solution, you can carry them out in a
polystyrene cup with a lid, and measure the temperature change using a
thermometer
what is an exothermic change or reaction
heat energy is given out
what is an endothermic change/ reaction
when heat energy is taken in
what type of reaction is breaking bonds
endothermic
what reaction is making bonds
exothermic
how do you know if the overall heat energy change for a recation is exothermic
if more heat energy is released in forming bonds
in the products than is required in breaking bonds in the
reactants
How do you know that the overall heat energy change for a reaction is endothermic
if less heat energy is released in forming bonds
in the products than is required in breaking bonds in the
reactants
Calculate the energy change in a reaction given the
energies of bonds (in kJ mol–1)
check book and cognito/lesson materials to practice
what is activation energy
the minimum amount of energy needed to start a reaction
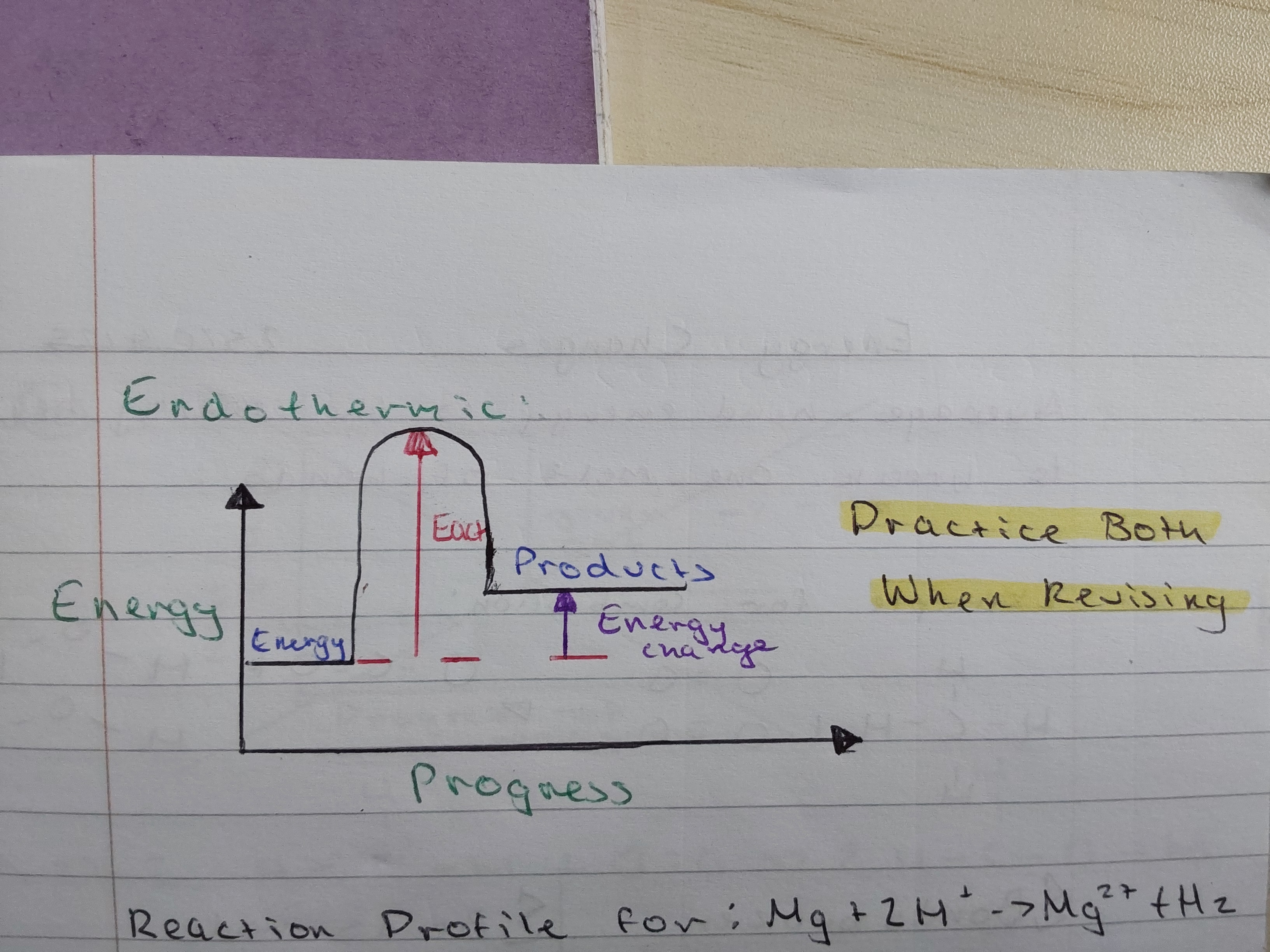
what does a reaction profile for endothermic reactions look like
what does a reaction profile for exothermic reactions look like
