A2 Substitution and Elimination
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
what does concerted mean
all bonds are made/broken at the same time
what is rate determined by for SN2
activation energy
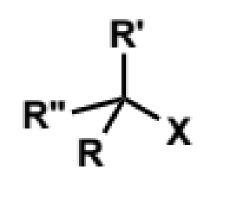
what are the important aspects for a leaving group X in a substitution reaction
the C-X bond breaking
the group X accepting the former bonding electrons to become a lone pair
what do good leaving groups have for SN2
weak C-X bonds and happy to accept electrons
low/negative pKa values (eg counterions of strong acids)
halides as leaving groups
the hydrohalic acids are mostly strong and hence they are quite good leaving groups. fluoride is NOT a leaving group.
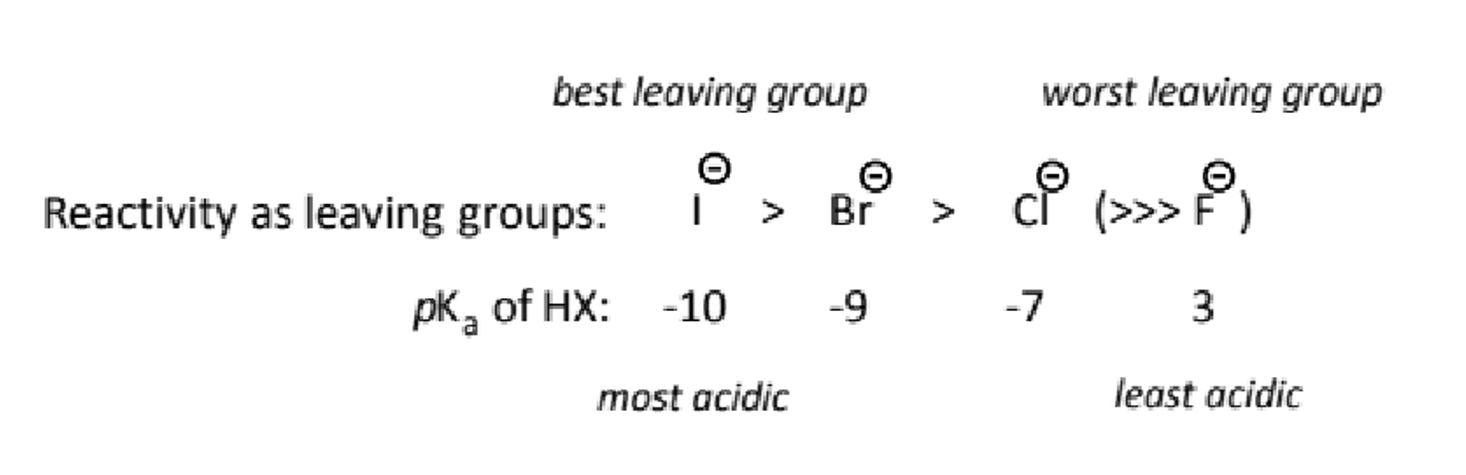
how are simple and complex halides made
simple - made by radical halogenation of alkanes
complex - made from alcohols
is hydroxide a good leaving group
no. alcohols cannot act as electrophiles in substitution reactions
the conjugate acid of hydroxide is water - not acidic, pKa (H2O) = 15. the leaving group property of the counterion (OH-) from the strong acid is not met.
how can alcohols be made into good leaving groups
when does this work
protonate them to make the leaving group water, whose conjugate acid is H3O+ - a strong acid. pKa = -3.
only works with a narrow range of non-basic nucleophiles eg Cl, Br, I. most nucleophiles are also good bases and will just react with the acid in a neutralisation reaction.
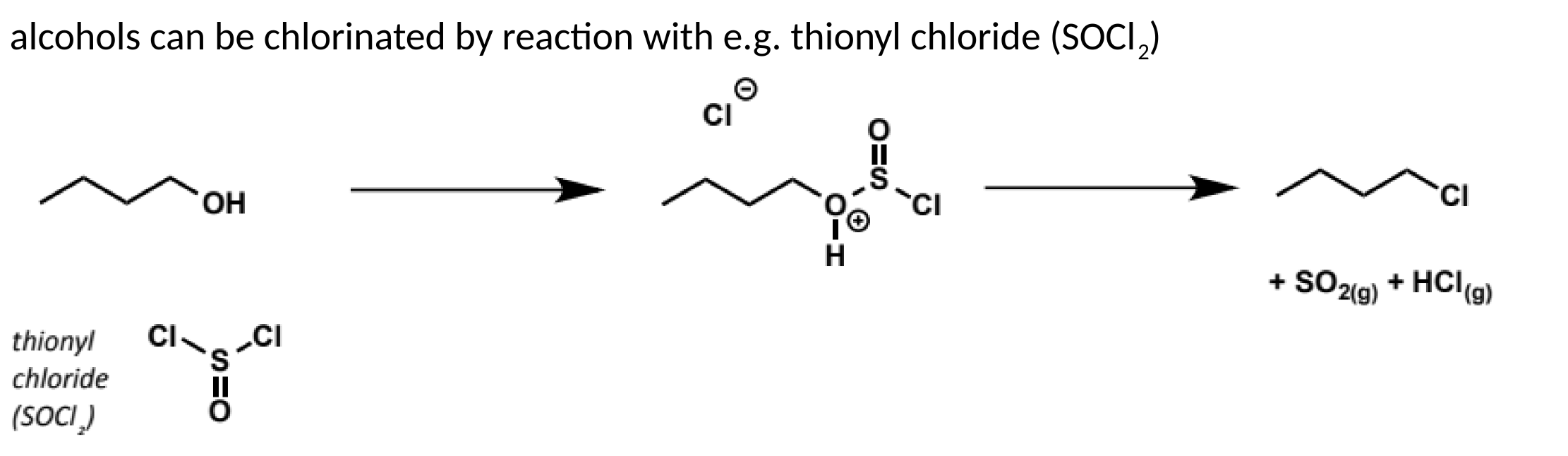
chlorination of alcohols
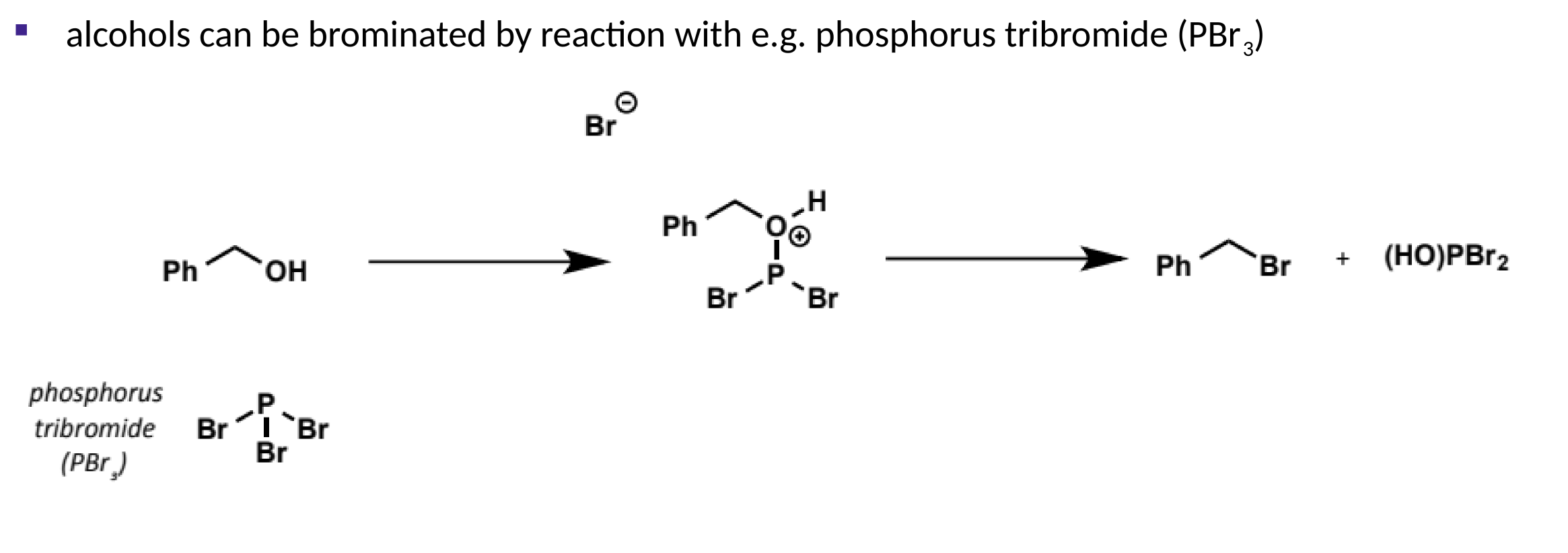
bromination of alcohols
what can be done to alcohols apart from making halides to turn them into good leaving groups
turn the into sulfonate esters
sulfonic acids are very strong (approx pKa 3) so sulfonate ions are excellent leaving groups
3 examples of sulfonate esters
para-toluenesulfonate (tosylate)
methanesulfonate (mesylate, MeSO3-, MsO-)
trifluoromethanesulfonate (triflate, CF3SO3-)
tosylate
para-toluenesulfonate
single bonded O not in Ts group
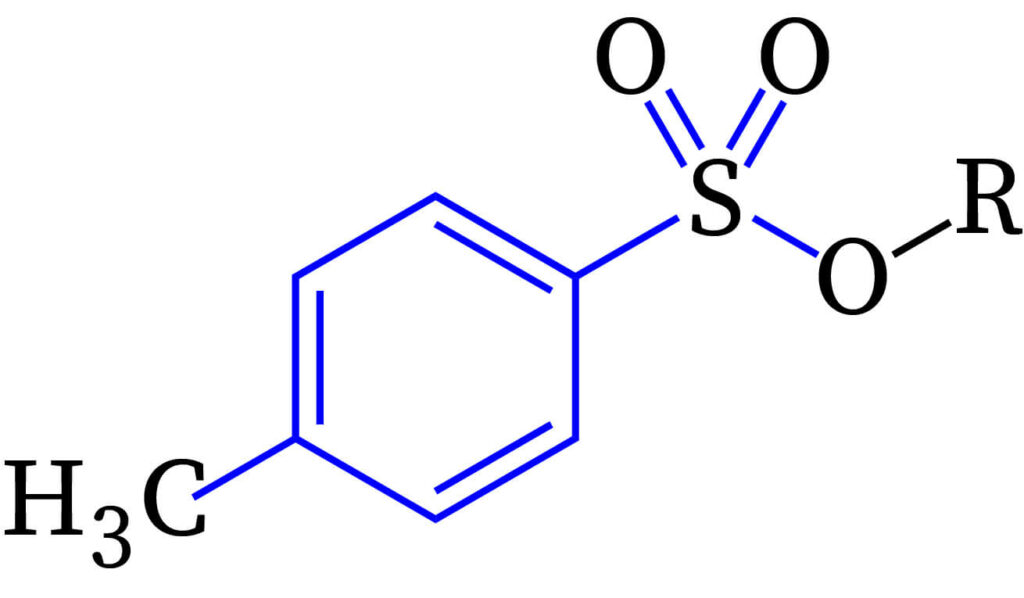
mesylate
methanesulfonate (MeSO3-, MsO-)
the single bonded O is indeed not part of the group :(

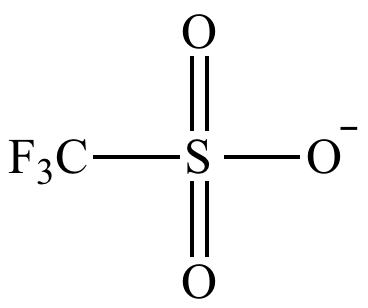
triflate group
trifluoromethanesulfonate (CF3SO3-)
single bonded O not in Tf group
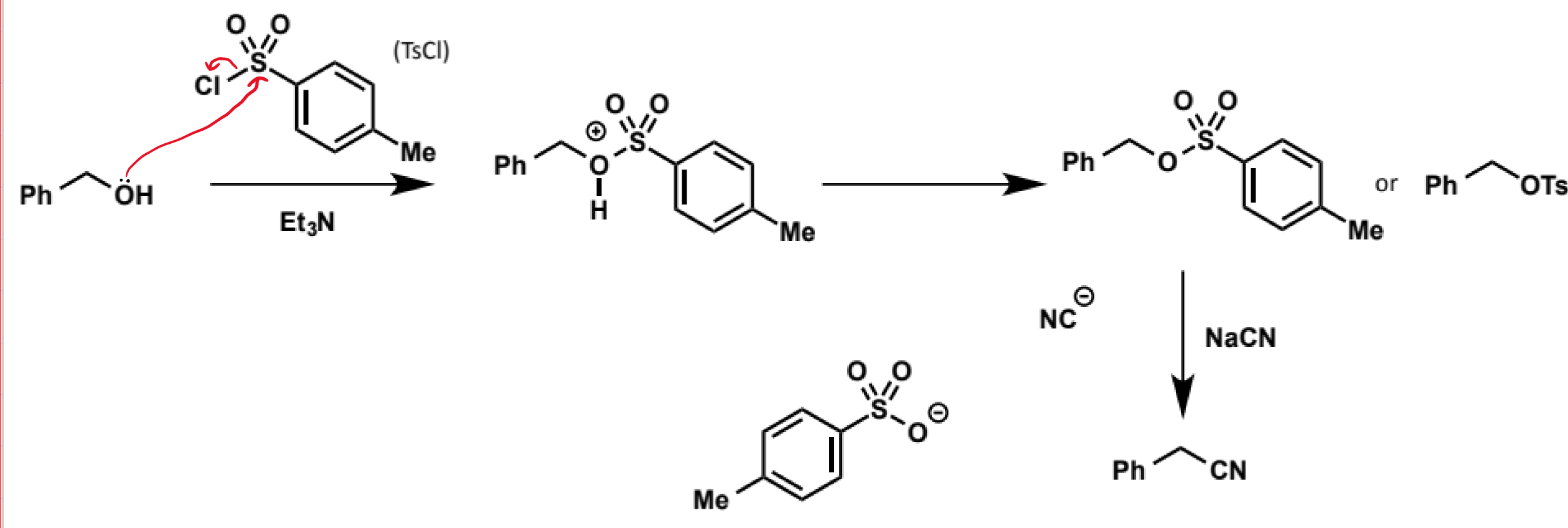
production of tosylate from phenol
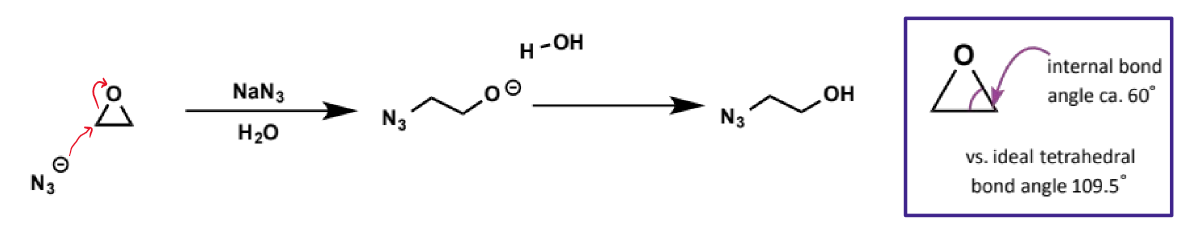
alkoxides as leaving groups
not good leaving groups - conjugate acids are alcohols (pKa 16-18) so cannot be used as SN2 electrophiles
exception to alkoxides as leaving groups
cyclic three-membered ethers (oxiranes/epoxides) are very good electrophiles despite the alkoxide being a poor leaving group
this is because the C-O bond is unusually weak because of the severe angle strain within the three-membered ring. the relief of this angle strain on opening of the epoxide provides the extra driving force to overcome the otherwise unfavourable formation of the alkoxide
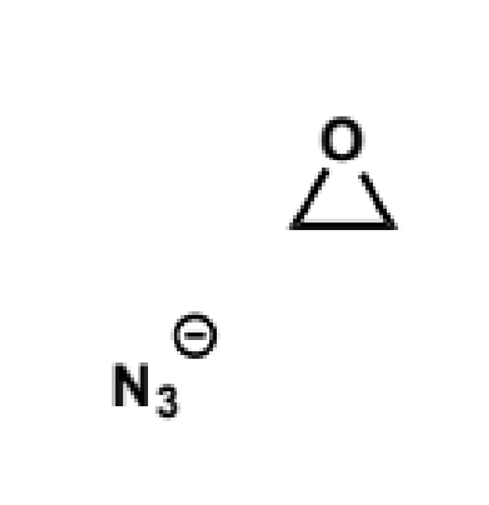
mechanism/products/reagents/conditions/explanation
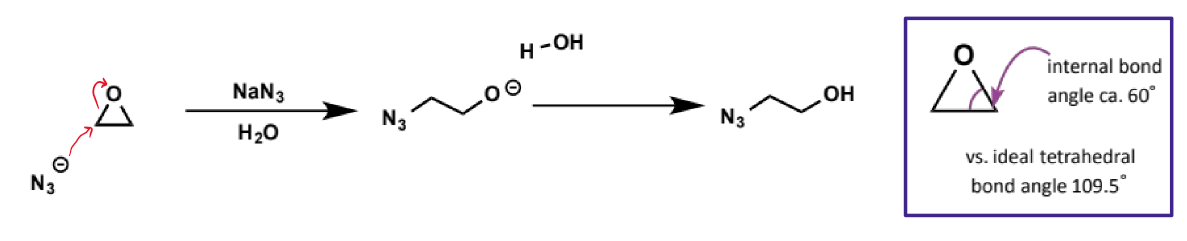
effect of substitution on the electrophile on SN2 reactivity
reactivity decreases with increasing substitution around the carbon undergoing attack (ie rate of reaction decreases across the series methyl > primary > secondary > tertiary alkyl group)
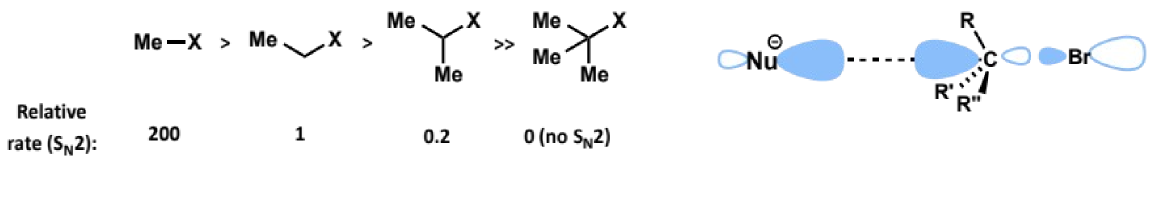
effect of neighbouring π systems in electrophile on SN2
orbitals? stages?
3 examples
rate is increased by presence of neighbouring π systems
the π system overlaps with the p-orbital on the carbon in the transition state, stabilising it and lowering the energy barrier. π systems are also EWG helping make the carbon more electrophilic and susceptible to nucleophilic attack.
this makes allylic, benzylic and α-carbonyl electrophiles good SN2 substrates
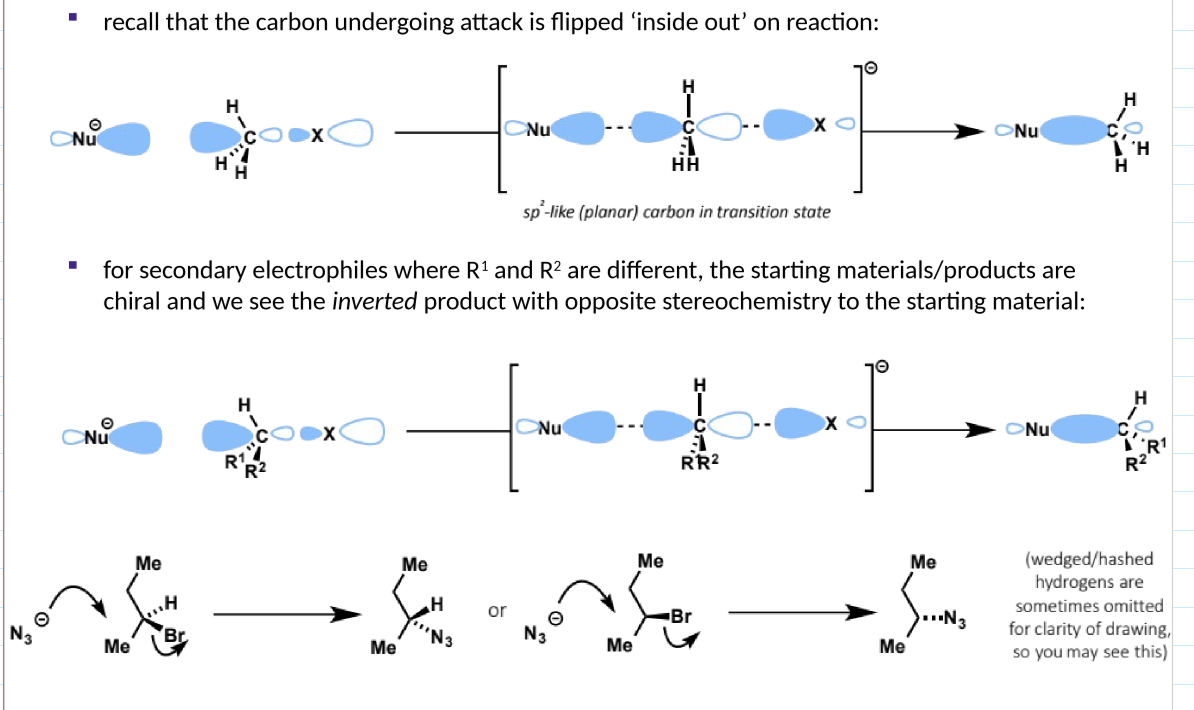
inversion of stereochemistry for SN2 reactions
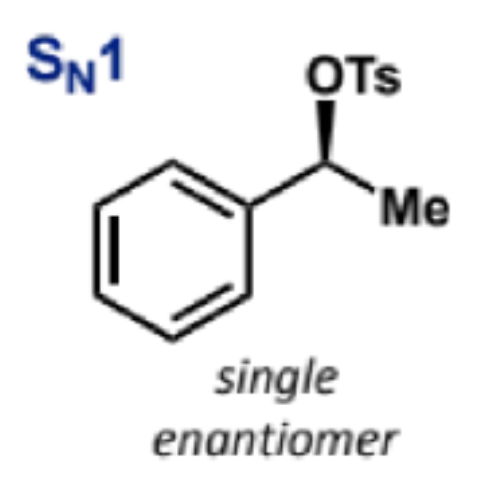
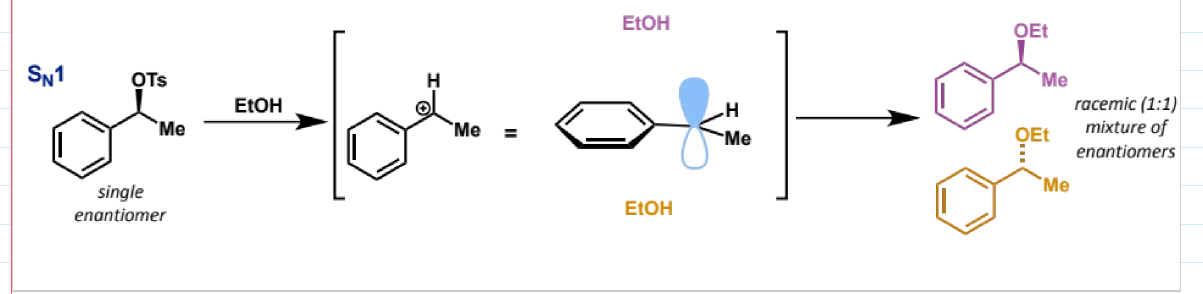
stereochemistry in SN1 reactions
with EtOH
a racemic (1:1) mixture of the two enantiomers is formed, as the stereochemistry of the starting material is lost in the planar carbocation intermediate, and this intermediate can be attacked from either face with equal likelihood, giving the 1:1 mixture
what makes a good nucleophile
high-lying HOMO (often meaning a readily donatable lone pair) allowing effective overlap with the LUMO of the electrophile (often C-X σ*)
better nucleophile of same element
for a given element, the more basic a species is, the better a nucleophile it is
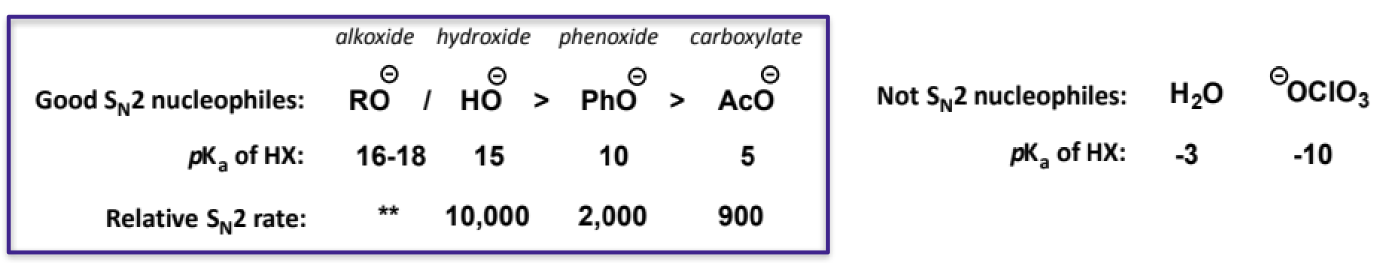
nucleophiles showing basicity condition for SN2 + examples that are not SN2 nucleophiles
water and perchlorate are basic but not nucleophiles
note about water and OH- pKas - the pKa for OH- is water’s pKa, while the pKa for water is H3O+’s, as these are their conjugate acids
limitations to basicity correlation
does not apply to nucleophiles formed from different elements (cannot compare N and O for example)
steric effects - the bigger a nucleophile is, the poorer it is
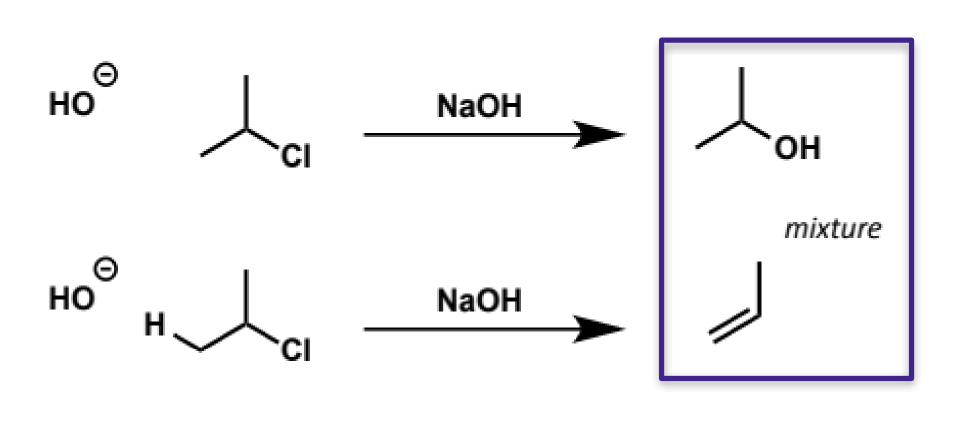
hydroxide as an SN2 nucleophile
hyroxide is a good SN2 nucleophile and can be used for example to form alcohols from alkyl halides
however, it is also a good base (pKa H2O = 15) so there is a competing reaction (E2). the balance between SN2 and E2 will depend on how good of an Sn2 electrophile the other reactant is (if it is not good SN2 will be slow and E2 will dominate)
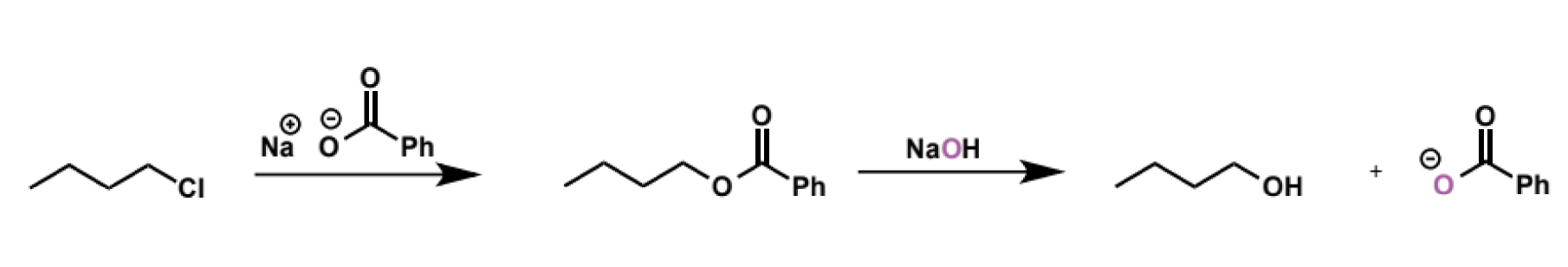
carboxylates as SN2 nucleophiles
good SN2 nucleophiles (less reactive than hydroxide but still highly competent)
because they are not basic (counterions of a weak acid) they do not cause complications with E2, and there is only SN2
this is used to perform 2 step conversion of alkyl halides to alcohols where the use of hydroxide is unsuitable due to E2. clean SN2 with carboxylate is followed by ester hydrolysis under basic conditions
2 methods to convert alkyl halide into alcohol
1 step with OH easier/faster except for competition with E2
where E2 is a problem, 2 step conversion with carboxylate is used
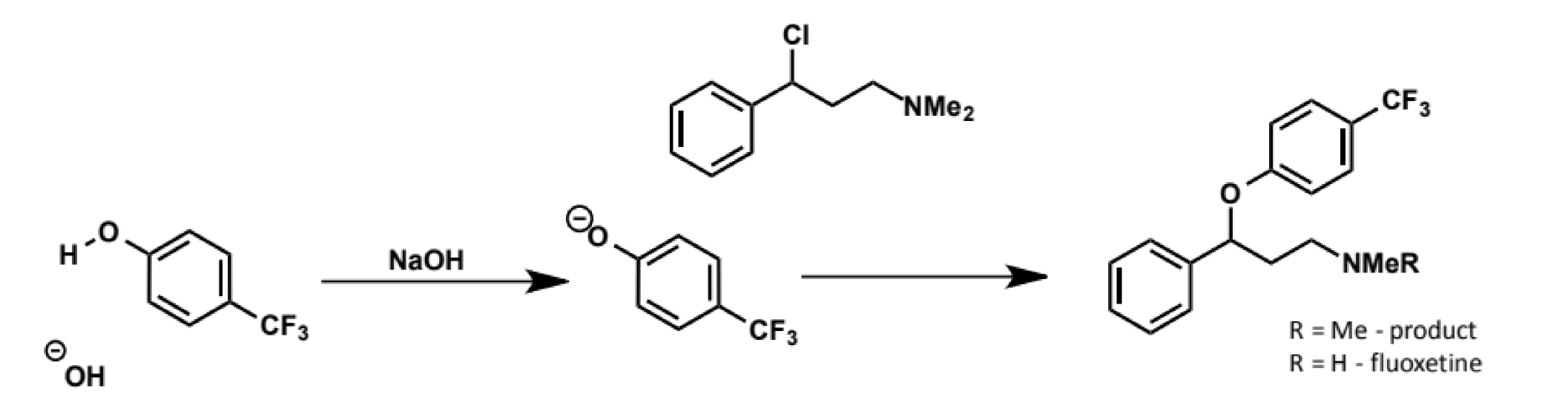
phenoxides as SN2 nucleophiles
anions of phenols (phenoxides) are good SN2 nucleophiles
they are only weakly basic (pKa of weak acids = ca. 10) so they do not cause complications with E2
how are phenoxides made
can prepare separately or
can make them in a single step by treating then phenol with a base like hydroxide (as PKa is approx 15 it can quickly and fully deprotonate then phenol) in the presence of the electrophile
this reaction is known as Williamson ether synthesis. it is widely used, eg with the above reaction synthesising the antidepressent fluoxetine (Prozac)
alkoxides as SN2 nucleophiles
anions of alcohols (alkoxides) are very good SN2 nucleophiles
as they are more basic than OH (OH pKa 15, alcohols 16-18) they can also cause problems with E2
SN2 and E2 competition
generally, if both nucleophile and electrophile (especially) are relatively non-hindered, SN2 will dominate. increasing hindrance on either increases E2
preparation of alkoxides
alcohols are not very acidic so the anions need to be pre-formed using a relatively strong base (OH not strong enough), sodium hydride is common
thiols as SN2 nucleophiles
can use to make sulfides (also known as thioethers) by SN2.
more acidic than alcohols (pKa PhSH = 6.5 vs PhOH = 10) so hydroxide fine as a base
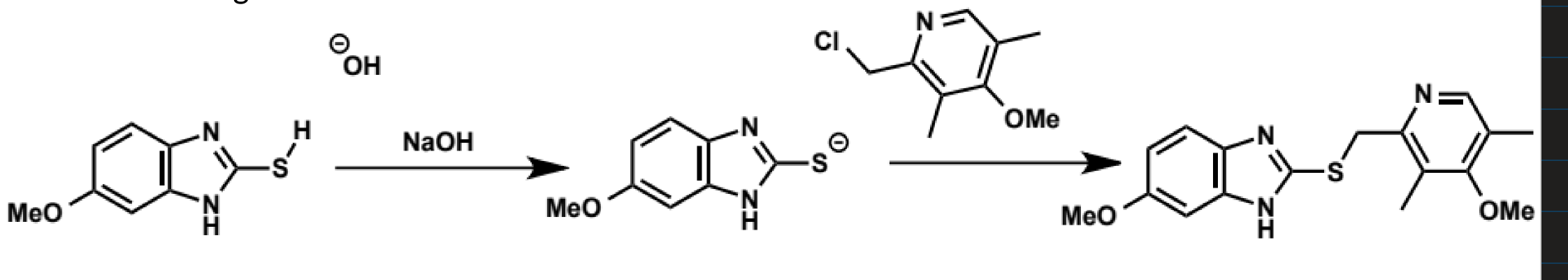
thiolates as SN2 nucleophiles
better nucleophiles than their oxygen equivalents (thiols) despite being less basic
PhS- is ca. 25,000 times more nucleophilic than PhO-)
what happens to nucleophilicity as you go down a group
nucleophilicity increases as higher energy HOMO is better matched to LUMO
mechanism + explanantion
SN2
nitrogen is a more basic atom (better lone pair donor) than oxygen and neutral amines are excellent SN2 nucleophiles
but it can be difficult to stop the reaction after one substitution and often different methods are used to make substituted amines
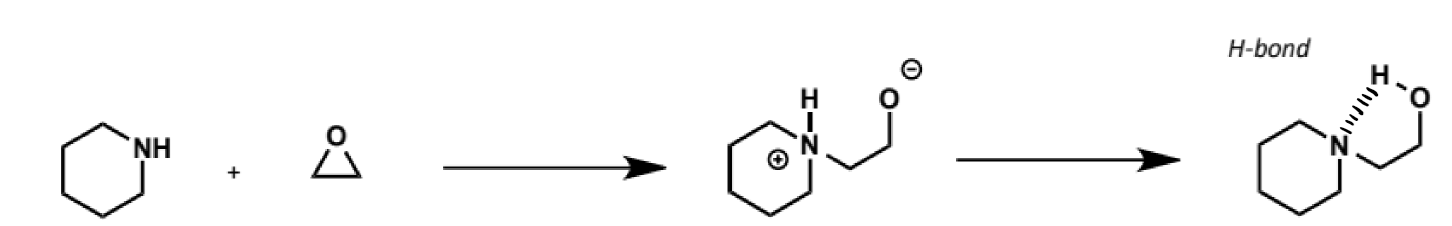
electrophile for SN2 with amine nucleophiles
epoxides
reactions are more easily controlled in terms of over-alkylation (nucleophilicity of amino alcohol product is attenuated by hydrogen bonding)
drug synthesis using amines as SN2 nucleophiles
1,2-aminoalcohols are found in many pharmaceuticals such as beta-blockers, asthma treatments and protease inhibitors
azide as SN2 nucleophiles
excellent SN2 nucleophile (small, linear molecule with negative charge on the nitrogen)
the azide function can also be reduced to give a primary amine, which is a much more reliable way of making amines through SN2 than by using ammonia as there is no possible overalkylation of the azide
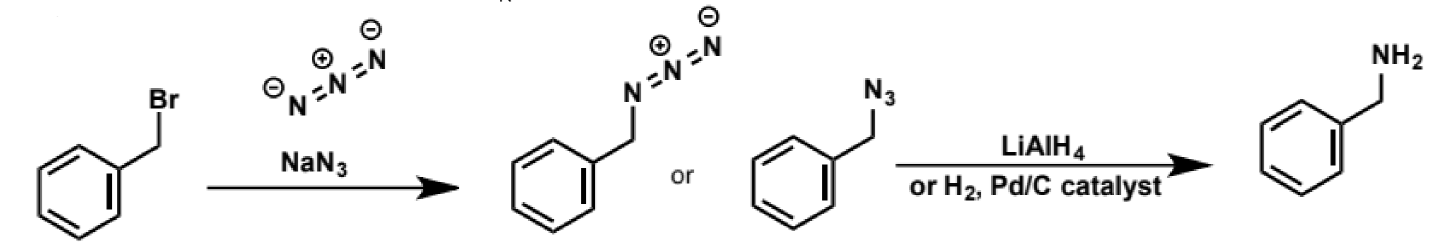
cyanide as an SN2 nucleophile
cyanide anion is a very good SN2 nucleophile (small, negatively charged on carbon)
products contain one more carbon than the starting materials - they have been homologated
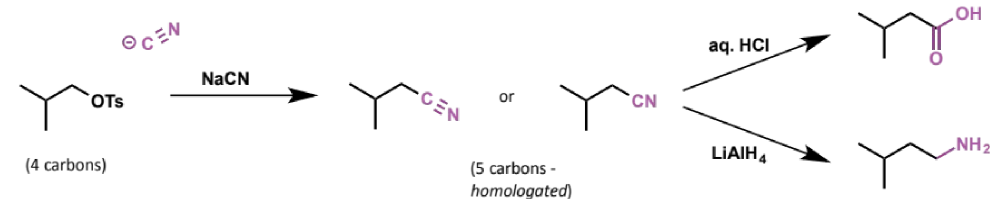
what is homologation
a process that turns the product into the next member of the homologous series - ie adds one carbon to the chain
what can cyanide groups be converted into
other useful groups eg carboxylic acids (by hydrolysis) or primary alkylamines (by reduction)
acetylide as an SN2 nucleophile
ethyne (acetylene) is relatively easy to turn into an anion known as acetylide. you can buy lithium acetylide, which is a good SN2 nucleophile
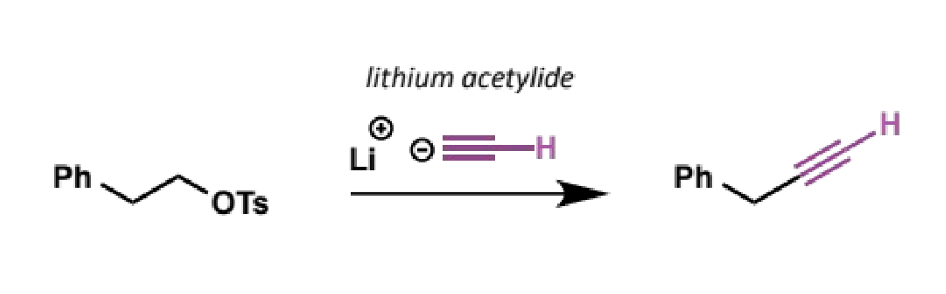
how are acetylide anions made
use a very strong base such as sodium amide
the resulting alkynes can be used to selectively make either E or Z alkenes (don’t need details for now)
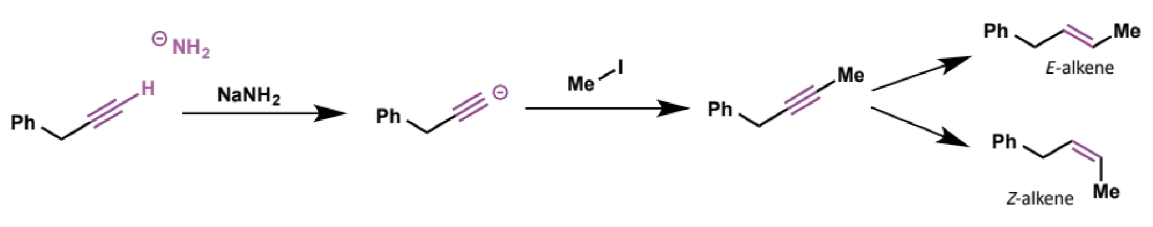
when can elimination occur instead of substitution
if a carbocation is generated but there is no nucleophile to react with it, a proton can be lost instead in an elimination reaction
E1 of C(Me)3X
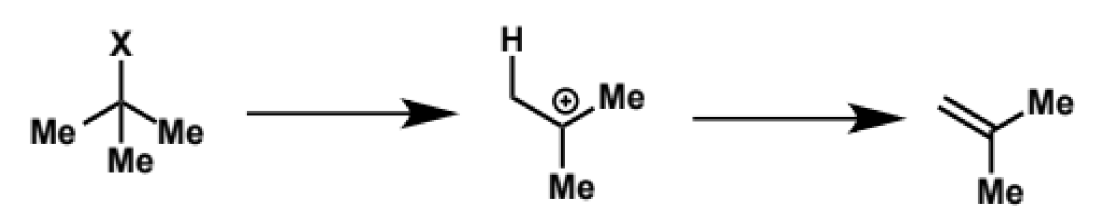
rate profile and rate equation for E1 of C(Me)3X
rate = k[XC4H9]
![<p>rate = k[XC<sub>4</sub>H<sub>9</sub>]</p>](https://knowt-user-attachments.s3.amazonaws.com/484ce4f0-6e71-4f64-a5c9-5634c82aff87.png)
kinetics of E1 and RDS
RDS is formation of high-energy cation so E1 follows unimolecular kinetics as SN1 does
E1 of tertiary alcohols
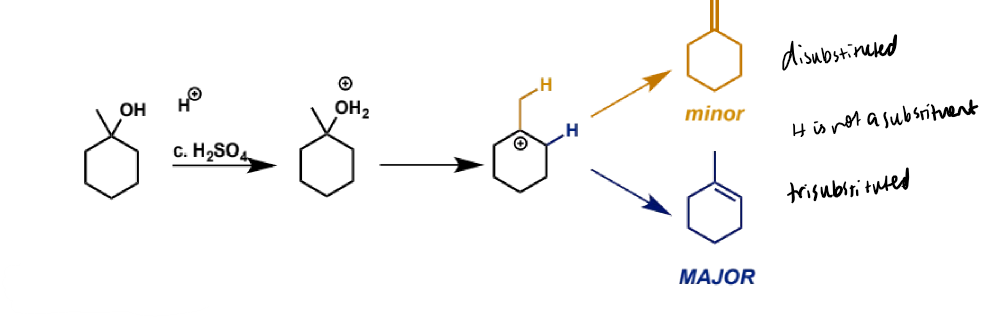
regiocontrol in E1
more than one alkene can be formed from intermediate carbocation
E1 tends to give the more substituted alkene as the major product
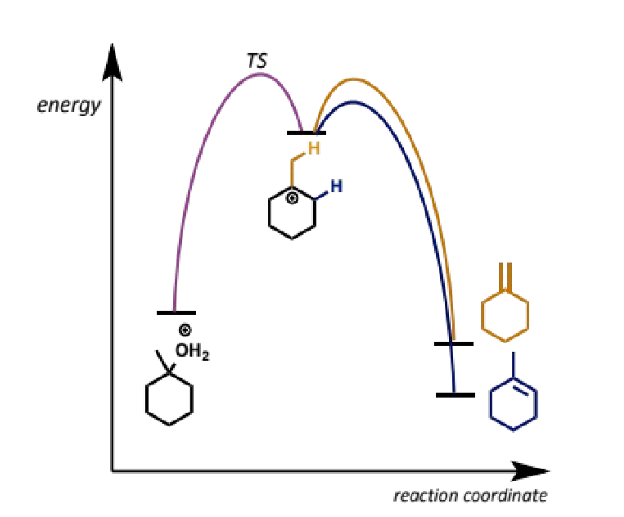
kinetics leading to regiocontrol in E1
more substituted is usually more stable product but also
pathway to the major product from the common intermediate is lower in energy than the pathway to the minor product so it proceeds faster
stereocontrol in E1
for some substrates, geometric isomers can be formed from the intermediate.
E1 gives the E-alkene as the major product
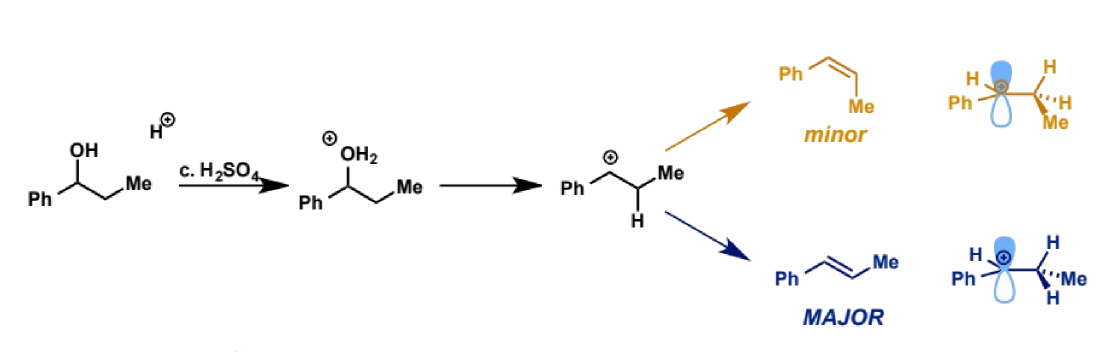
kinetics leading to stereocontrol in E1
the E-alkene is more stable than the Z but also
pathway to E from common intermediate is lower in energy and hence faster than Z
E2 of isopropyl chloride
kinetics of E2 (type, rate profile, description, RDS)
base and electrophile both RDS (one step)
bimolecular, concerted, single-step, all bonds broken and formed at same time
rate = k[ClC3H7] [OH-]
![<ul><li><p>base and electrophile both RDS (one step)</p></li><li><p>bimolecular, concerted, single-step, all bonds broken and formed at same time</p></li><li><p>rate = k[ClC<sub>3</sub>H<sub>7</sub>] [OH<sup>-</sup>]</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/fc25688e-aa69-4cd7-adbd-ca9ab24dea07.png)
good electrophiles for E2
unlike the other 3 mechanisms, E2 is not too fussy about electrophile structure
as long as there is an abstractable proton on the next carbon which can adopt suitable reactive shape (see stereo)
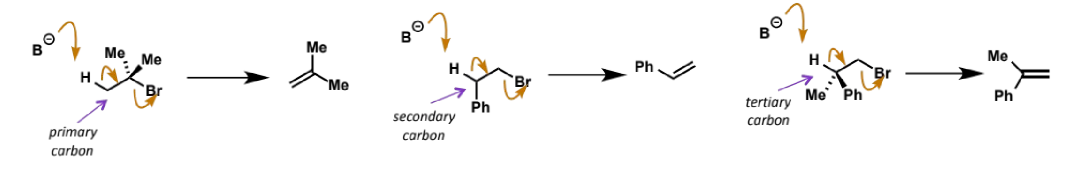
good leaving groups for E2
same as SN2
so halides, sulfonate esters like tosylates, epoxides
NOT protonated alcohols - incompatible with the base
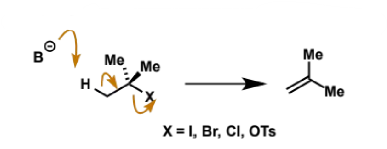
bases for E2
as seen in SN2, many nucleophiles are also basic and cause mixtures of SN2 and E2
can get clean E2 with either tertiary alkyl halides (no SN2) or very bulky bases which don’t act as nucleophiles

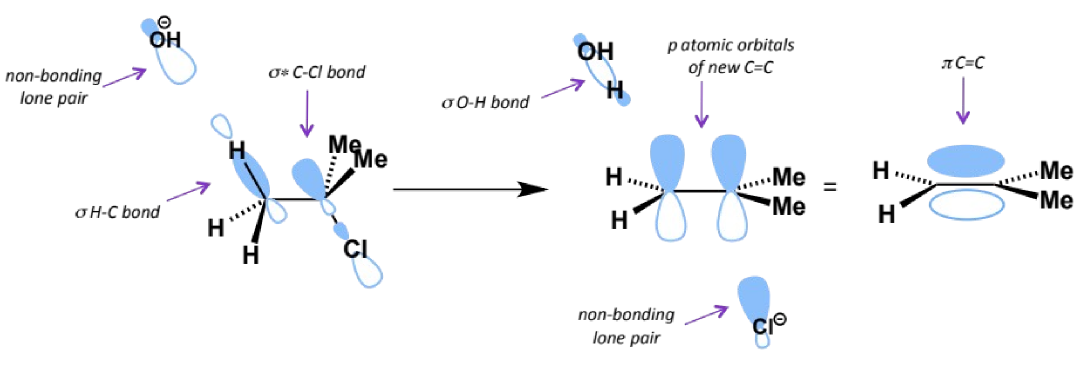
stereoelectronics of E2
as with SN2, because all bonds are formed and broken at once, the arrangement of the reacting orbitals in space must be considered to ensure the electrons can reorganise smoothly into the new bonding orbitals
most favourable arrangement of E2 has C-H breaking at 180° to the C-X breaking, which maximises overlap of the breaking C-H bond with C-X σ*, and pushing electrons into this breaks the C-X bond
this arrangement is called anti-periplanar and only this formation can react to form a product (see example E2 from another flashcard - the orange product does not form)
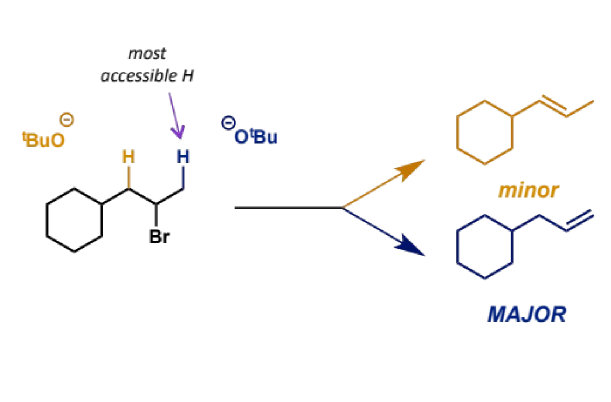
regiochemistry in E2
where there is more than one possible site for a proton to be abstracted in E2, we find the major product is the less substituted alkenes
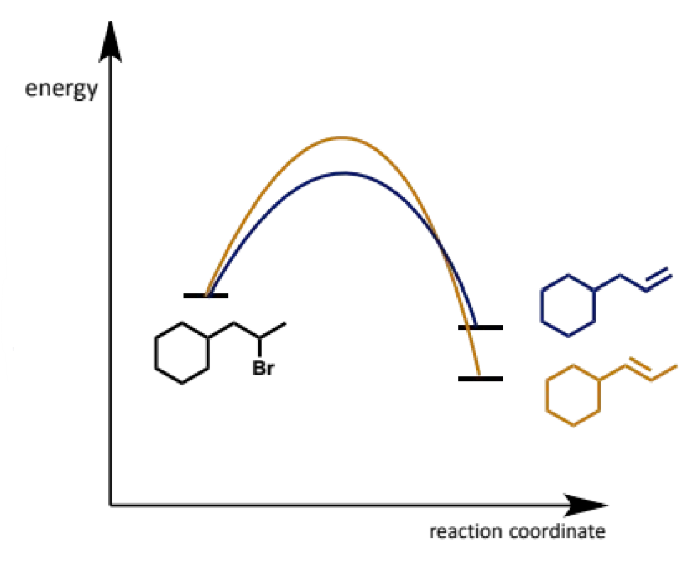
kinetics leading to regiochemistry in E2
the less substituted alkene is actually the less stable alkene
it arises because the transition state for attack on the more accessible proton is lower in energy than the more crowded transition state for attack at the less accessible proton
it is useful that this is the complementary regiochemical outcome to E1
stereochemistry in E2
where two possible alkene stereoisomers are possible, the E alkene is the major product
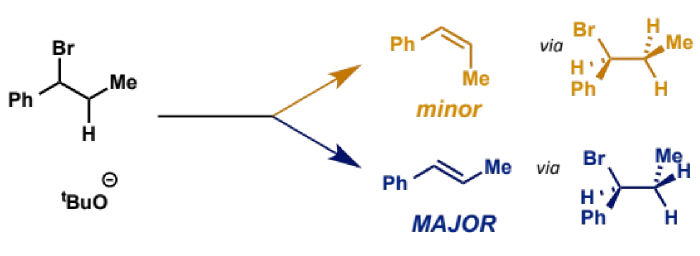
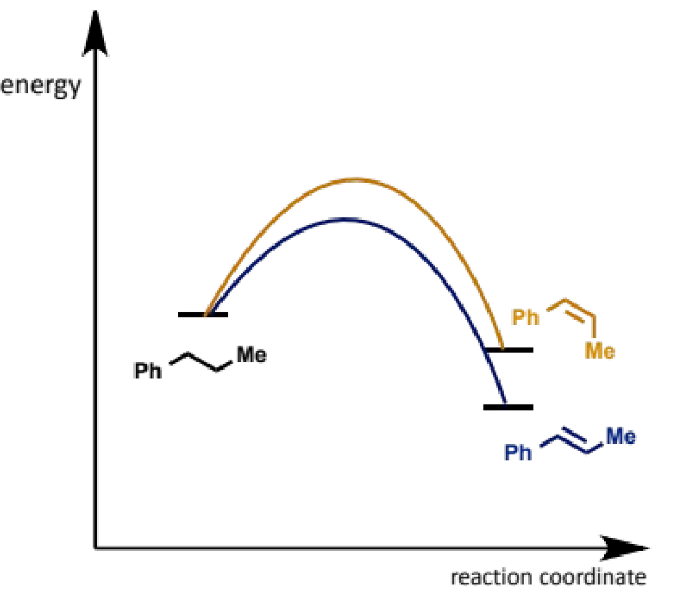
kinetics leading to stereochemistry of E2
E alkene is most stable but again is not why it forms
it is the faster formed product because the transition state leading to it is less crowded than for Z
this means both E1 and E2 favour E and so Z must be made by other means
methyl group summary for sub&elim
SN2 with good nucleophiles, else no reaction
primary alkyl group summary for sub&elim
SN2 with good nucleophiles
SN1 if R is alkene or aromatic
E2 with strong base

secondary alkyl group summary for sub&elim
SN2 with good nucleophiles
SN1 with weaker nucleophiles
E2 with strong base
E1 with poor bases or in acid
tertiary alkyl group summary for sub&elim
SN1 with any nucleophiles
E2 with strong base
E1 with poor bases or in acid
NO SN2