Interphase Chemistry - Week 2
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
The Periodic Table Organization
Rows → Periods; Columns → Groups
○ Elements in same group have similar chemical
properties
Bonding
Core electrons: electrons in the inner shells.
Don’t play a major role in chemical bonding
Valence electrons: electrons in the outer shell.
Plays a major role in chemical bonding
Valence electrons are in non-filled shells*
Coulomb’s Law
“Effective Nuclear Charge” (Zeff): The positive charge actually felt by the negative electron by the nucleus
Farther away from the nucleus, the less attractive interaction the electron feels
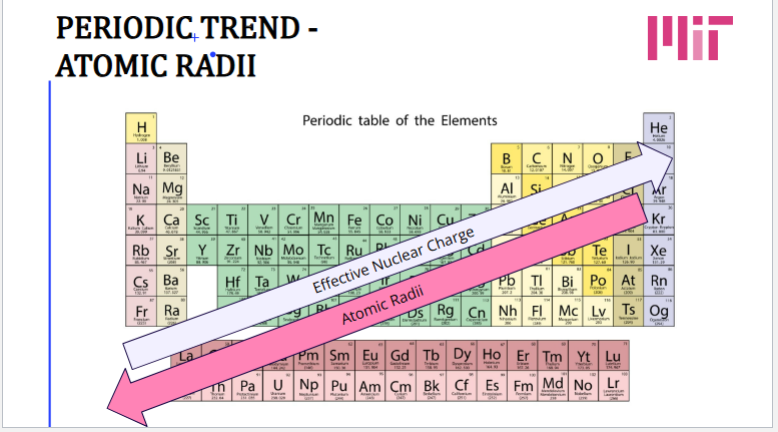
Ionic Radii
Trend:
● Cations are always smaller in radius
● Anions are always larger in radius
Isoelectronic series:
● More protons → smaller radius
● Less protons → larger radius
Ionization Energy
The amount of energy necessary to remove the
highest-energy electron from an isolated neutral atom in the gaseous
state
X(g) → X+(g) + 1e-
ΔE = IE1
Covalent Bond
occurs when there is equal force of attraction
between two nuclei on two or more electrons (ie. Zeff is identical)
Polar Covalent Bond
occurs when there is unequal attraction of
electron pairs between two nuclei.
○ This occurs when there is a difference in electronegativity
between the two elements (Zeff)
ionic bond
formed through the coulomb attraction of two ions of
opposite charge. It is formed when the electronegativities of the two
atoms is significantly different (usually greater than 1.7)
BOND STRENGTH & LENGTH
Strength: The energy required to separate the atoms is called the
bond strength (or the bond energies)
○ covalent < polar covalent < ionic
● Bond Length: As the atoms increase in size, so will the bond lengths
DIPOLE MOMENT
When there is a difference in electronegativity between the two elements
that make up the polar covalent bond, there is an uneven distribution of the
electrons
DIPOLE MOMENT &
% IONIC CHARACTER
In reality, there is not a complete transfer of electrons from one atom to the next.
○ There is a partial transfer, which leads to the formation of the dipole moment
(μ)
Equation for Dipole Moment:
δ= fraction of a unit charge on each atom in a diatomic molecule
R(Å) = distance separating atoms (in angstroms)
We can take δ(100) = % ionic character

Harpoon Mechanism
t’s far-fetched and
only applies to 6 compounds, but
trust us--it works.
● Describes mechanics for bond
formation
For this model we assume*:
● Ionic bonds involve a complete* transfer of (1+) electrons
between two atoms
● Bonding results from the electrostatic attraction between the
cation and the anion
COULOMBIC INTERACTION
This is the energy of interaction between two ions and can be
quantified by the following equation:
Explanation of the symbols:
Q = charges on the ions (which is the Unit charge, e (magnitude of charge), for instance Na+1,
Q = 1 (1.60217646 x 10-19 C)
ε₀ = permittivity of the vacuum, proportionality constant with a value of 8.854 x 10-12 C2 J-1 m
r = distance separating the charges ( in m)
Units for ΔEcoulomb are J/molecule, to convert to kJ/mole, the results need to be multiplied
it by Na (Avogadro's #) and divide by 103 (convert from J → kJ)
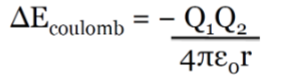
Steps to find empirical formula of compound given weight of compounds
1. Convert masses to moles:
For each element in the compound, divide its mass by its molar mass (found on the periodic table).
Example: If you have 10 grams of element X with a molar mass of 20 g/mol, you would have 10/20 = 0.5 moles of X.
2. Find the mole ratio:
Divide the number of moles of each element by the smallest number of moles calculated in the previous step.
Example: If you have 0.5 moles of element X and 1 mole of element Y, you would divide both by 0.5: 0.5/0.5 = 1 for X and 1/0.5 = 2 for Y. This gives a mole ratio of 1:2.
3. Determine the empirical formula:
The mole ratios you obtained in the previous step become the subscripts for each element in the empirical formula.
steps to calculate the minimum mass of a product
1. Balance the Chemical Equation:
Ensure the number of atoms for each element is the same on both sides of the equation. This provides the mole ratios for the reactants and products.
2. Determine the Limiting Reactant:
Convert the given masses of reactants into moles by dividing by their respective molar masses.
Divide the number of moles of each reactant by its corresponding coefficient in the balanced equation.
The reactant with the smallest result is the limiting reactant, as it will be consumed first.
3. Calculate Moles of Product:
Use the mole ratio from the balanced equation to relate the moles of the limiting reactant to the moles of the desired product.
For example, if the ratio is 2:1 (two moles of reactant produce one mole of product), and you have 0.5 moles of the limiting reactant, you will produce 0.25 moles of product.
4. Convert Moles to Mass:
Multiply the moles of the product by its molar mass to find the minimum mass of the product that can be formed