autoimmunity and hypersensitivity
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
In Brief: Hypersensitivity is…
Excessive or inappropriate immune response to antigens
Can be towards harmless foreign antigens (allergy, microbiota) or self (autoimmunity)
Classified as Type I – IV
allergies vs autoimmunities
hypersensitivity can be towards harmless foreign antigens (allergy, microbiota) or self (autoimmunity)
In Brief: Autoimmunity
Type of hypersensitivity
Immune response against self-antigens
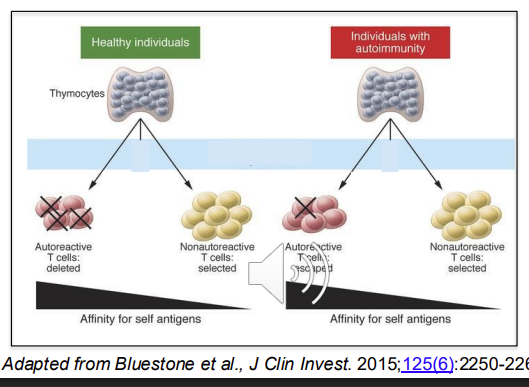
Failure of tolerance mechanisms - usually to self antigens, highly reactive T cells are aoptosed in negative selection
Leads to chronic inflammation, tissue injury
Involves innate and adaptive immunity
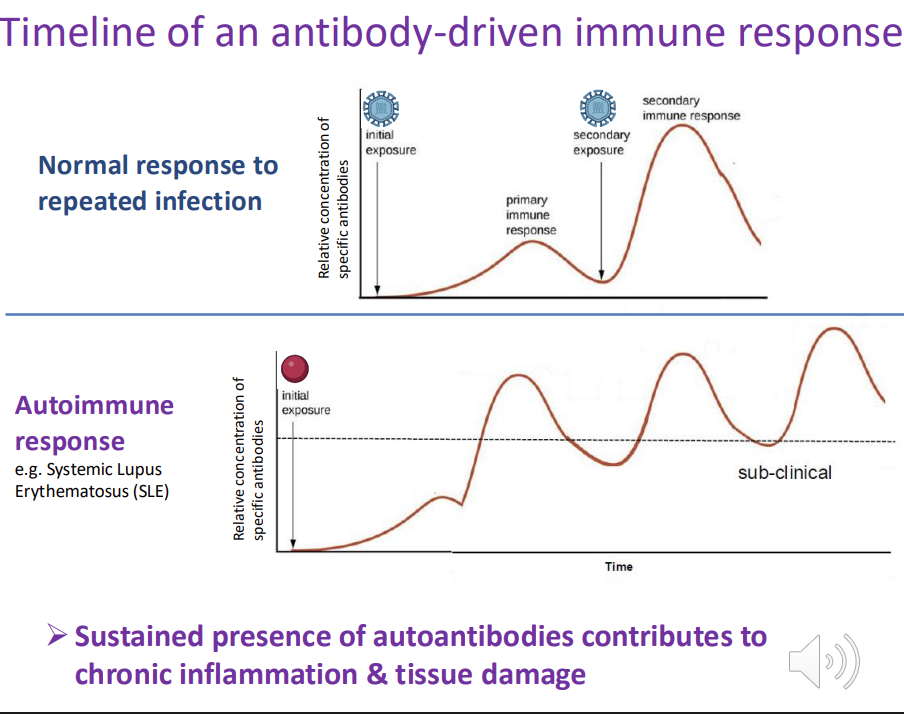
Timeline of an antibody-driven immune response - explain the graph
autoimmune response - antibodies are produced against a self-antigen
antibodies fail to return to low/initial levels after the initial response → chronic inflammation and ongoing tissue damage
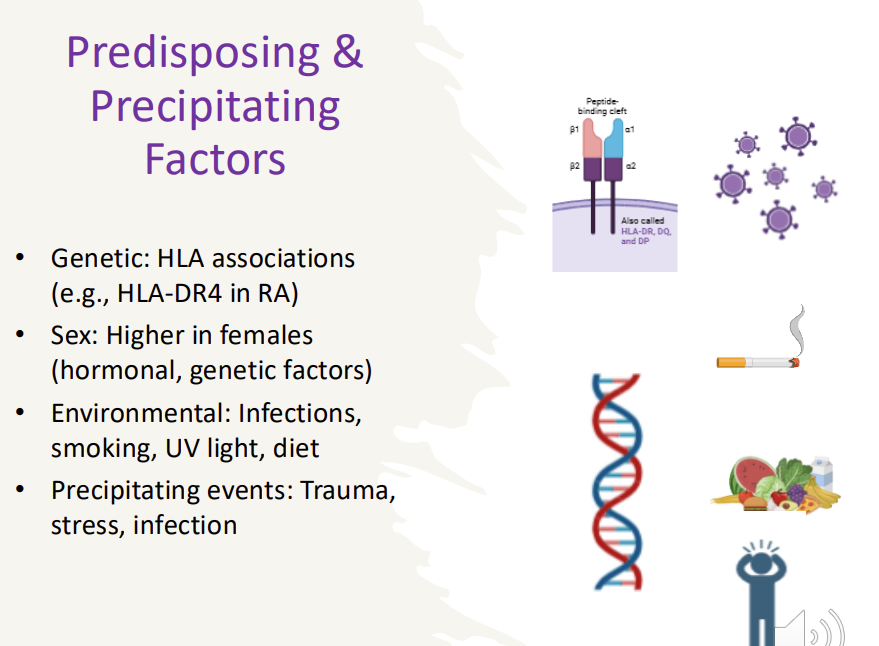
Predisposing & Precipitating Factors to autoimmunity
Genetic: HLA associations (e.g., HLA-DR4 in RA)
Sex: Higher in females (hormonal, genetic factors)
Environmental: Infections, smoking, UV light, diet
Precipitating events: Trauma, stress, infection
autoimmunity - overview steps 5
loss of central tolerance
defects in peripheral tolerance
environmental triggers/precipitating events
activation of autoreactive lymphocytes
amplification and chronic inflammation
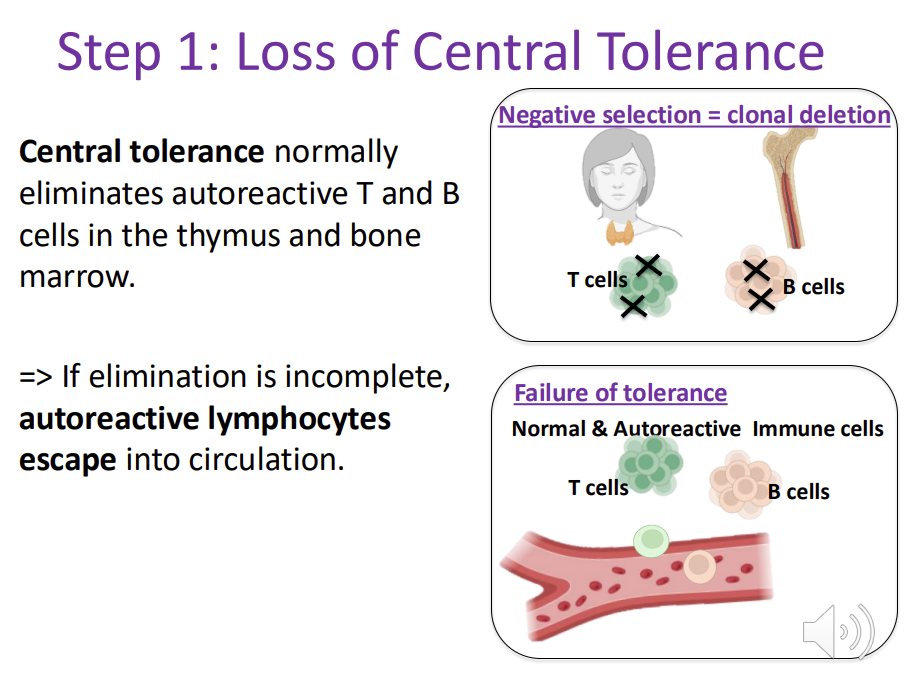
Autoimmunity - Step 1: Loss of Central Tolerance
Central tolerance normally eliminates autoreactive T and B cells in the thymus and bone marrow. => If elimination is incomplete, autoreactive lymphocytes escape into circulation.
Mechanisms that normally keep escaped autoreactive cells under control: 3
Anergy: autoreactive lymphocytes fail to activate → lost.
Regulatory T cells (Tregs): suppress autoreactive responses.
Deletion (apoptosis): elimination of activated autoreactive cells.
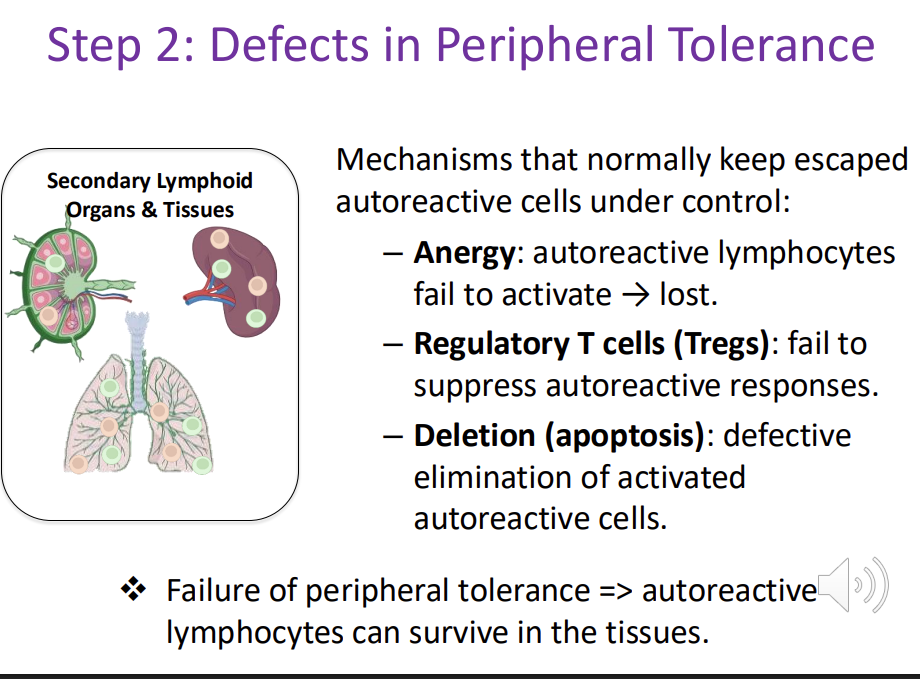
Autoimmunity - Step 2: Defects in Peripheral Tolerance (back up mechanism)
Mechanisms that normally keep escaped autoreactive cells under control:
Anergy: autoreactive lymphocytes fail to activate → lost.
Regulatory T cells (Tregs): fail to suppress autoreactive responses.
Deletion (apoptosis): defective elimination of activated autoreactive cells.
Failure of peripheral tolerance => autoreactive lymphocytes can survive in the tissues
Autoimmunity - Step 3: Environmental Triggers / Initiating Events
External factors provide the “spark” that activates autoreactive cells:
Infections (molecular mimicry, bystander activation) — e.g., rheumatic fever after Streptococcus infection.
Tissue damage — release of normally hidden (“sequestered”) antigens.
Drugs, toxins, UV light — can alter self-antigens, making them appear foreign.

Autoimmunity - Step 4: Activation of Autoreactive Lymphocytes
Under the influence of costimulatory signals - like TCR binding to MHC (from infection or inflammation), autoreactive T or B cells become activated.
These cells start producing pro-inflammatory cytokines or autoantibodies.

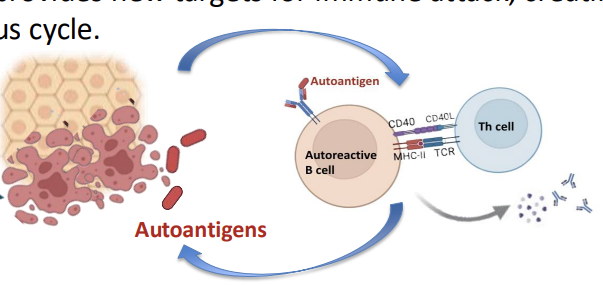
Autoimmunity - Step 5: Amplification and Chronic Inflammation
Once initiated, the immune response becomes selfperpetuating:
Autoantigens are continuously released from damaged tissue.
This provides new targets for immune attack, creating a vicious cycle
Chronic inflammation leads to progressive tissue damage and clinical disease.
Normal vs Autoimmune Responses

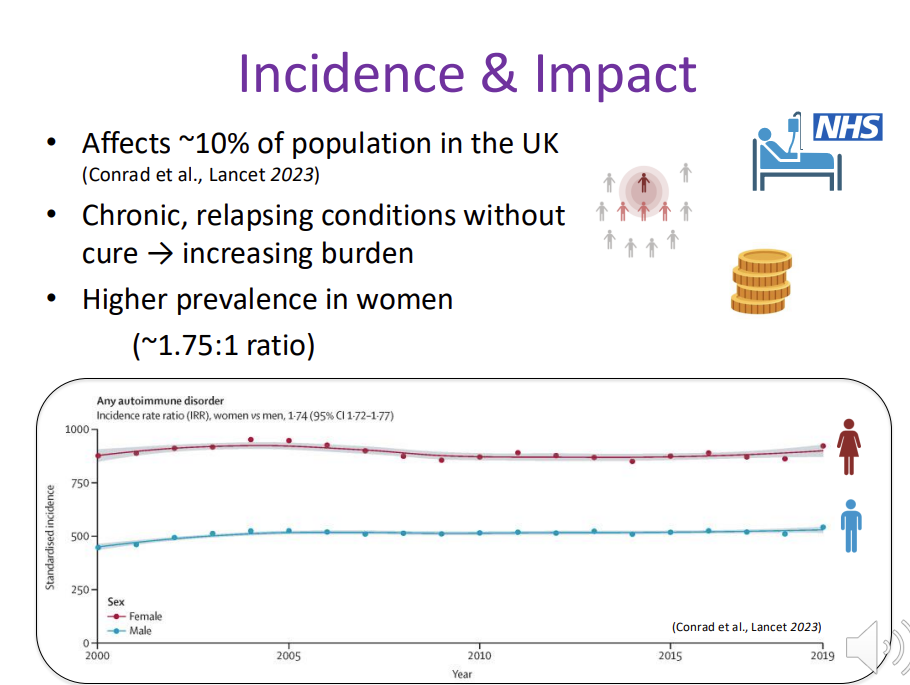
autoimmunity - Incidence & Impact
Affects ~10% of population in the UK (Conrad et al., Lancet 2023)
Chronic, relapsing conditions without cure → increasing burden
Higher prevalence in women (~1.75:1 ratio)
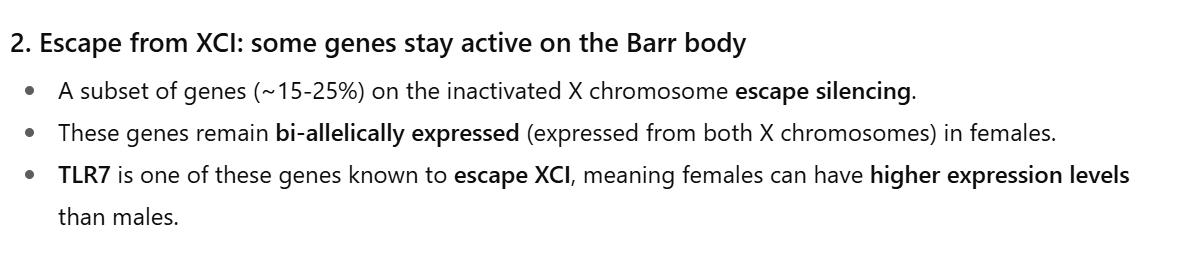
Sex-Divergence in Autoimmunity
Majority of autoimmune disorders are more common in women Reasons:
Estrogen enhances (autoreactive) B cell survival & antibody production
X-chromosome genes (e.g., TLR7 duplication in SLE)
Pregnancy/hormonal shifts modulate disease activity

Organ-Specific Autoimmune Diseases - 3
affect a particular organ or tissue type, with localised damage and dysfunction
Type 1 diabetes - immune system attacks the insulin-producing beta cells in the pancreas - typically develops in childhood or adolescence - genetic factors (e.g., HLA-DR3 and DR4 alleles) & environmental triggers (e.g., viral infections
Multiple sclerosis - affects the central nervous system (CNS) - immune system targets the myelin sheath of nerve fibres - often begins in early adulthood- muscle weakness, vision problems and co-ordination issues
Hashimoto’s thyroiditis - common cause of hypothyroidism (underactive thyroid) - immune system attacks the thyroid gland, impairing hormone production - tends to run in families, i.e. genetic factors are likely involved - fatigue, weight gain. cold intolerance and depression
Organ-Specific Autoimmune Diseases - diagnosis and treatment
Autoimmune responses can cause very targeted damage of organs, despite the immune system’s systemic role in protecting the body.
Diagnosis often involves detecting autoantibodies specific to the affected organ and using imaging or functional tests to assess organ damage.
Treatments to reduce inflammation, manage symptoms, replace lost function (e.g., insulin therapy in T1D or thyroid hormone replacement in Hashimoto’s).
Major side effect: immunosuppression
Systemic Autoimmune Diseases - 3
affect multiple organs and tissues throughout the body
Sjögren’s syndrome -autoimmune responses attack salivary and tear glands, leading to dry mouth and dry eyes. -involves multiple organs (lungs, kidneys, liver) nervous system -arises from a combination of genetic susceptibility and environmental triggers - fatigue, joint pain and swelling of the salivary glands
Systemic lupus erythematosus (SLE) - autoantibodies form immune complexes that deposit in tissues (skin, kidneys, joints, blood vessels) and trigger inflammation & tissue damage - disproportionately affects women, particularly during reproductive years - caused by combination of genetic susceptibility, hormones & environmental factors - joint pain, fatigue, rash
Rheumatoid arthritis (RA) - primarily affects the lining of the joints/synovium, leading to chronic inflammation, pain, swelling, and eventual joint destruction - also affects other organs, including the lungs, heart, and blood vessels - influenced by genetic and environmental factors (e.g. smoking) - fatigue, amenia and nodules under the skin
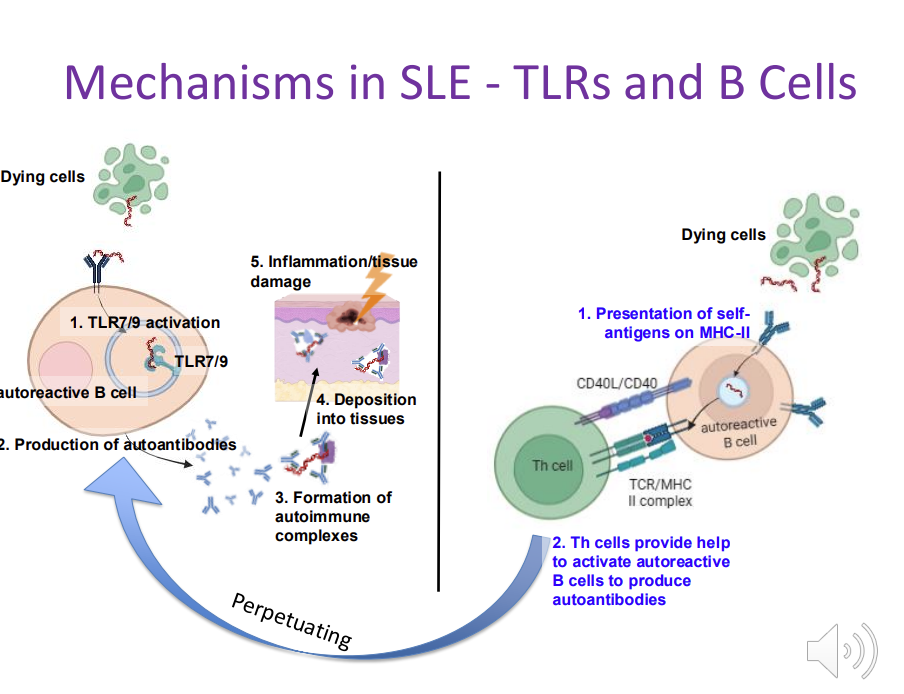
Mechanisms in SLE - TLRs and B Cells
TLR receptors that usually detect bacterial/viruses become activated when they encounter self-nucleic acids when they encounter dying cells
autoreactive B cells produce large amounts of autoantibodies
autoimmune complexes depositing in the skin
autoreactive B cells also act as APC - capture self antigens MHC II - activate autoreactive T cells
Mechanisms in RA - complex interplay of genetics, immune dysregulation, and microbiota 4
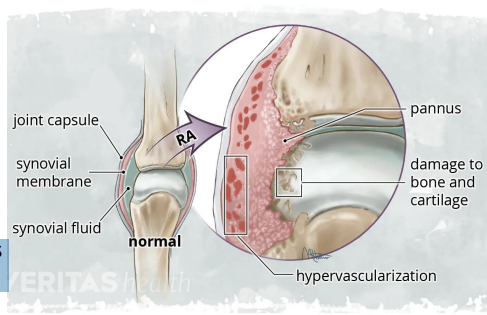
Joint Pathology - Autoimmune responses targets the synovium resulting in pannus formation (abnormal fibrovascular tissue), joint deformity & loss of function
joint pain lasting more than 1 hour- symmetrical joint pain and swelling
Genetic Factors - HLA-DR4 / HLA-DRB1 shared epitope alleles influence antigen presentation
Autoantibodies - Rheumatoid Factor (RF): antibody against Fc portion of IgG - Anti-CCP antibodies: highly specific, appear years before symptoms
Immune Cell Contributions
CD4+ T cells: release cytokines (TNF-α, IL-1, IL-6) that drive inflammation
B cells: produce autoantibodies & act as antigen-presenting cells
Macrophages: amplify cytokine production
Osteoclasts: mediate bone erosion
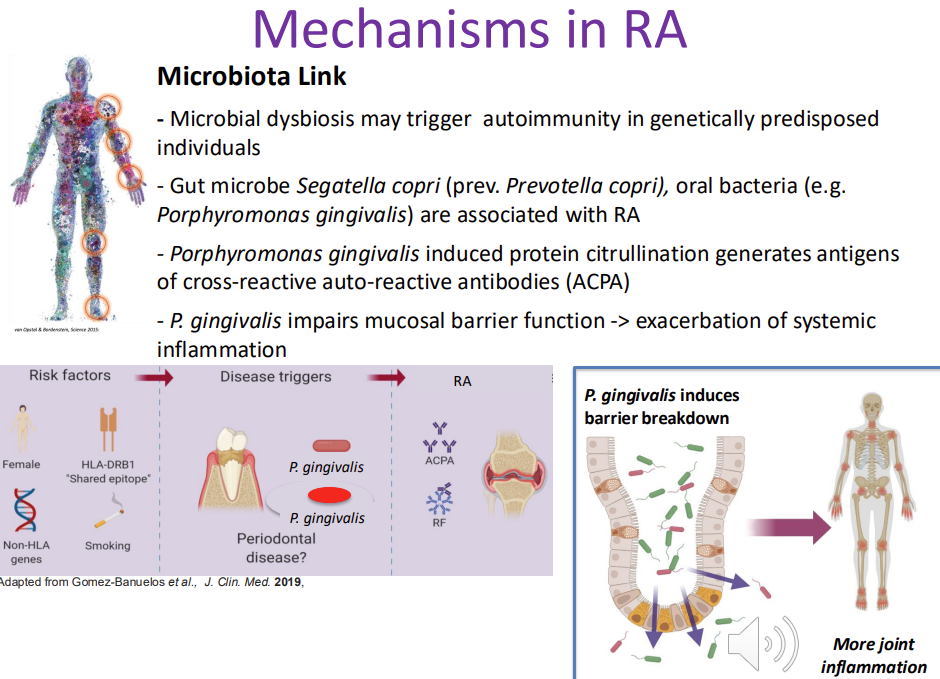
Mechanisms in RA → Microbiota Link
Microbial dysbiosis may trigger autoimmunity in genetically predisposed individuals
Gut microbe Segatella copri (prev. Prevotella copri), oral bacteria (e.g. Porphyromonas gingivalis) are associated with RA
Porphyromonas gingivalis (perio) induced protein citrullination generates antigens of cross-reactive auto-reactive antibodies (ACPA) - unique enzyme in P gingivalis can modify host proteins in a specific manner - citrillation - generates antigens targeted by CCP antibodies - drives RA
P. gingivalis impairs mucosal barrier function -> exacerbation of systemic inflammation
Comorbidities in systemic autoimmune diseases
Individuals with any autoinflammatory disease: increased risk for other comorbidities
Treatment: target multiple organs & systems simultaneously, while preserving functions of protective immune responses & other body functions

Hypersensitivity - how many types are there?
4
1 - immediate IgE mediated allergy
2 - antibody mediated cytotoxicity
3- immune complex diseases - RA, SLE
4- T cell mediated delayed response - contact derm T1D
Hypersensitivity - Type I - overview and examples
Mediated by IgE bound to high-affinity Fc receptors on mast cells, basophils, and eosinophils
Antigen cross-linking causes rapid degranulation (minutes)
Release of histamine, serotonin, leukotrienes, proteases
early and late phase
Examples: allergies (hay fever, asthma, food allergy);
not organ specific
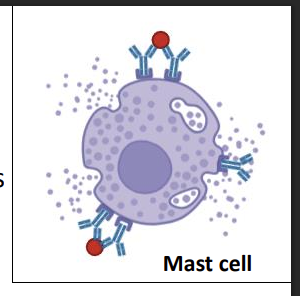
hypersensitivity type 1 early phase 3
(minutes)
Early phase effects (minutes):
increased vascular permeability
Smooth muscle contraction,
bronchoconstriction
swelling, itching and wheezing
hypersensitivity type 1 late phase (hours)
Late phase (hours):
cytokine release → recruitment of eosinophils, neutrophils, macrophages
extreme reaction = anaphylactic shock
Hypersensitivity - Type II (2-24h)
antibody mediated
Mediated by IgG (IgG1, IgG2, IgG3) binding to antigens on cell or tissue surfaces
- Effector mechanisms:
IgG Fc binds Fcγ receptors on macrophages & NK cells → cytotoxicity
IgG activates complement → opsonisation & phagocyte recruitment
Outcome: degranulation or phagocytosis, causing tissue damage
Typically organ-specific (selfantigen on target tissue)
Examples: autoimmune hemolyticanemia, Goodpasture’s syndrome, myasthenia gravis
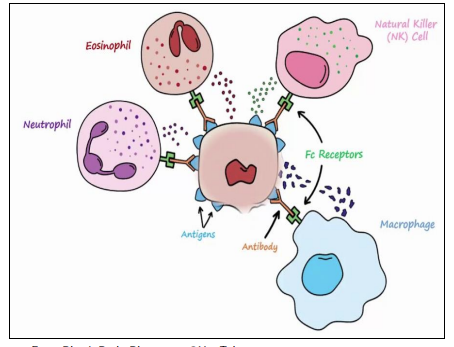
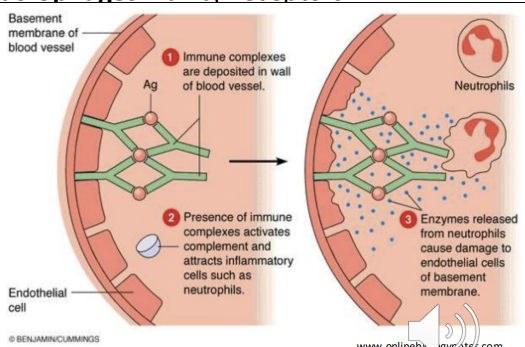
Hypersensitivity - Type III (hours-days)
immune complex
Mediated by IgG binding to soluble antigens → immune complex formation -
normally cleared but can acculate - Deposition of complexes in blood vessels, joints (synovial fluid), and other tissues -
Complement activation → release of C3a, C5a (inflammation, chemotaxis) -
Recruitment of neutrophils & macrophages via Fcγ receptors - Mast cell activation and vascular perimability - more leukocytes to area
Tissue damage
Non–organ-specific autoimmune diseases
Examples: SLE, RA, post-streptococcal glomerulonephritis)
Hypersensitivity - Type IV (DTH, 2-3 days)
Mediated by T cells (Th1, Th17, Tc) – not antibodies
Activated T cells secrete cytokines and chemokines IFN-γ, TNF → recruit & activate macrophages
Macrophages release IL-12, TNF → amplify inflammation - Tissue damage from macrophage degranulation & cytotoxic T cells
Chronic response → granuloma formation (macrophages, giant cells, eosinophils, T cells, fibroblasts)
Organ-specific autoimmune diseases
Examples: type 1 diabetes, contact dermatitis, multiple sclerosis; also chronic infections (e.g., TB)

Novel therapeutic approaches
Resolution of Inflammation
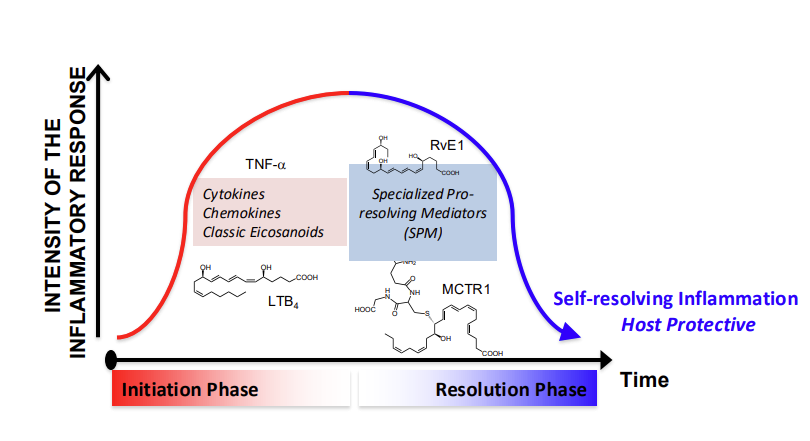
Specialized Pro-Resolving Mediators (SPMs)
Derived from omega-3 fatty acids (resolvins, protectins, maresins)
Actively resolve inflammation without suppressing immunity - unlike immunosuppressants
Reprogram white blood cells
Reduce neutrophil infiltration, enhance clearance of debris

What happens if Resolution fails?
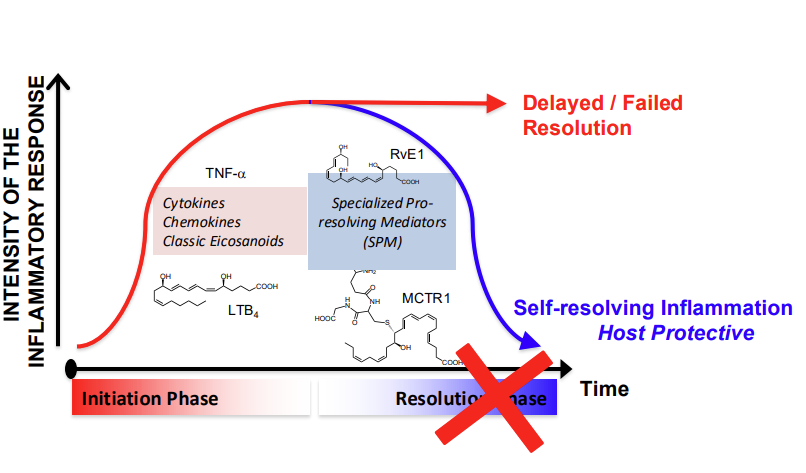
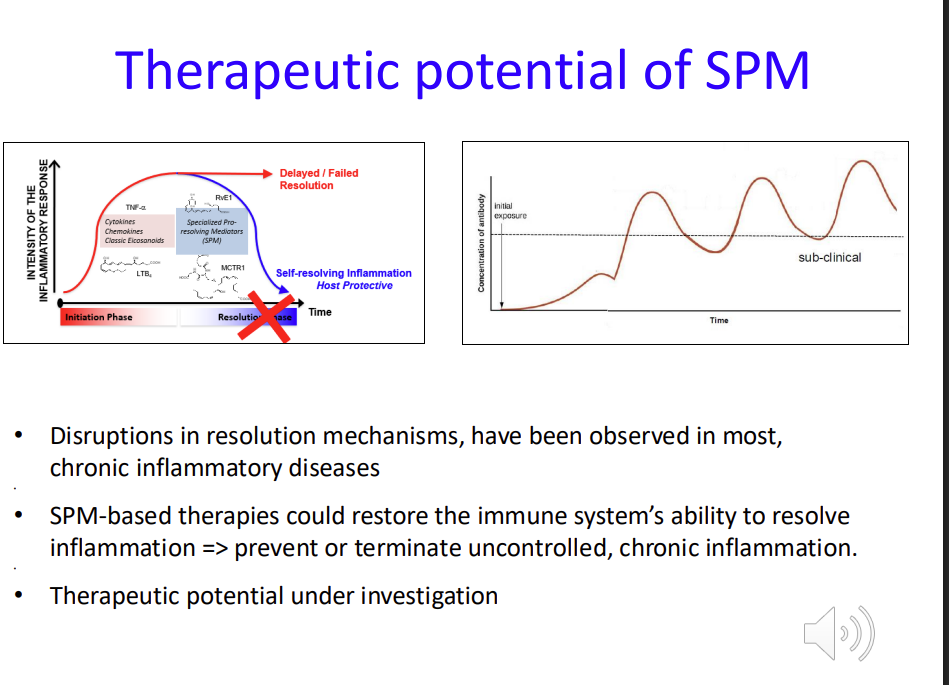
Therapeutic potential of SPM (Specialized Pro-Resolving Mediators)
Disruptions in resolution mechanisms, have been observed in most, chronic inflammatory diseases
SPM-based therapies could restore the immune system’s natural ability to resolve inflammation => prevent or terminate uncontrolled, chronic inflammation
Therapeutic potential under investigation