Reactive Oxygen Species
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
93 Terms
Example: Asphyxiation, what will happen to blood pH
Decrease in blood pH because of low oxygen

Example: Asphyxiation, What happens to glycolysis
Glycolysis increases, anaerobic, end with lactic acidosis

Example: Asphyxiation, What happens to the TCA cycle
Inoperable, high NADH

Hypoxia
lack of oxygen
What does CO poisoning block
Blocks hemoglobin of the ETC and O2 usage, blocks oxidative phosphorylation
Hypoxia can be caused by what events
Acute anemia
Ischemia asphyxiation
What is glycolysis activated by in lactic acidosis
Low ATP
TCA cycle in lactic acidosis
Inoperable, high NADH
How is pyruvate diverted to lactate
Acetyl CoA accumulates; PDH shut down; pyruvate diverted to lactate
Where is lactate sent
To circulation
Question: What type of proteins are induced by hypoxia
Glycolytic
Example: Hypoxia during Ischemic cell injury, myocardial infarction
Blood clot in the coronary arteries
Stops blood supply to the heart
Often, part of the heart muscle dies
Depleted ATP, accumulated ADP and NADH
Blocks TCA cycle
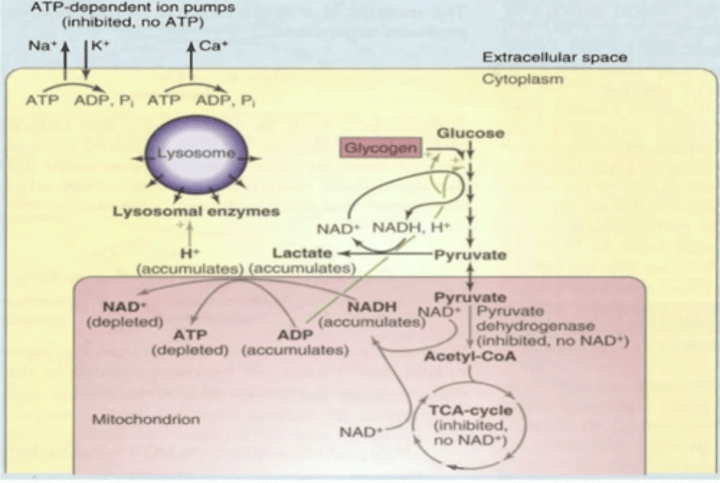
ATP is critical for maintaining what
Ionic radiance of calcium, sodium, and potassium
What happens when you break down ionic radiance of Ca++, Na+, and K+
Bad osmolar things happening leading to cell-lysis, RBCs susceptible to this because of glycolysis dependency
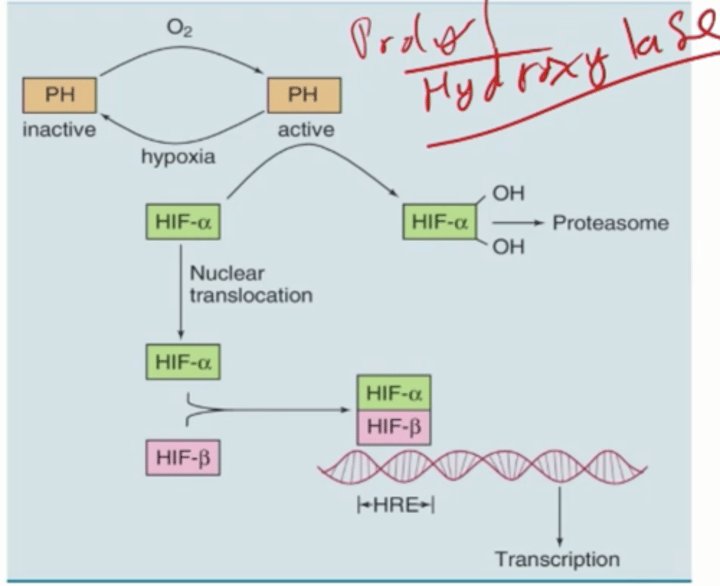
Adaptation to Hypoxia: Induction of HIF
Regulated by levels of oxygen
Have oxygen sensors: prolyl hydroxylase (PH)
more O2 = more PH
Hydroxylate HIF-alpha
Eating up by Proteasome (destroyed)
(not destroyed) Goes to nucleus
Binds to HIF-Beta
Forms regulatory complex
Increase transcription of glycolytic enzymes
Where are prolyl hydroxylases important
HIF
Connective tissue
Collagen
How are mitochondrial myopathies present
Muscles myopathies (weakness)
Neurological sx due to impaired mitochondrial function
When do mitochondrial myopathies pop up
Spontaneously, since they have their own DNA
What does MELAS stand for
Mitochondrial myopathy, Encephalopathy, Lactic acidosis, and Stroke-like episodes
What does MERRF stand for
Myoclonic epilepsy and Ragged red fibers
MELAS and MERRF result from what
maternally inherited defects in genes encoding mitochondrial tRNAs
What are MELAS
Poorly performing mitochondria that don't produce enough ATP, affecting brain and muscle
Mutation rate of Mitochondrial DNA
Much faster than nuclear DNA, increasing chance of mitochondrial myopathies from mitochondrially encoded genes
Why is oxygen dangerous (ROS)
Forms free radicals that are highly reactive

superoxide
O2 -1
Hydroxyl radical
Worst molecule, Highly reactive, reacts with anything near by, short
3 enzymes critical for regulating ROS
Superoxide dismutase
Catalase
Glutathione Peroxidase
Superoxide damages
Damages biomolecules (lipids, proteins, DNA)
Forms other reactive species
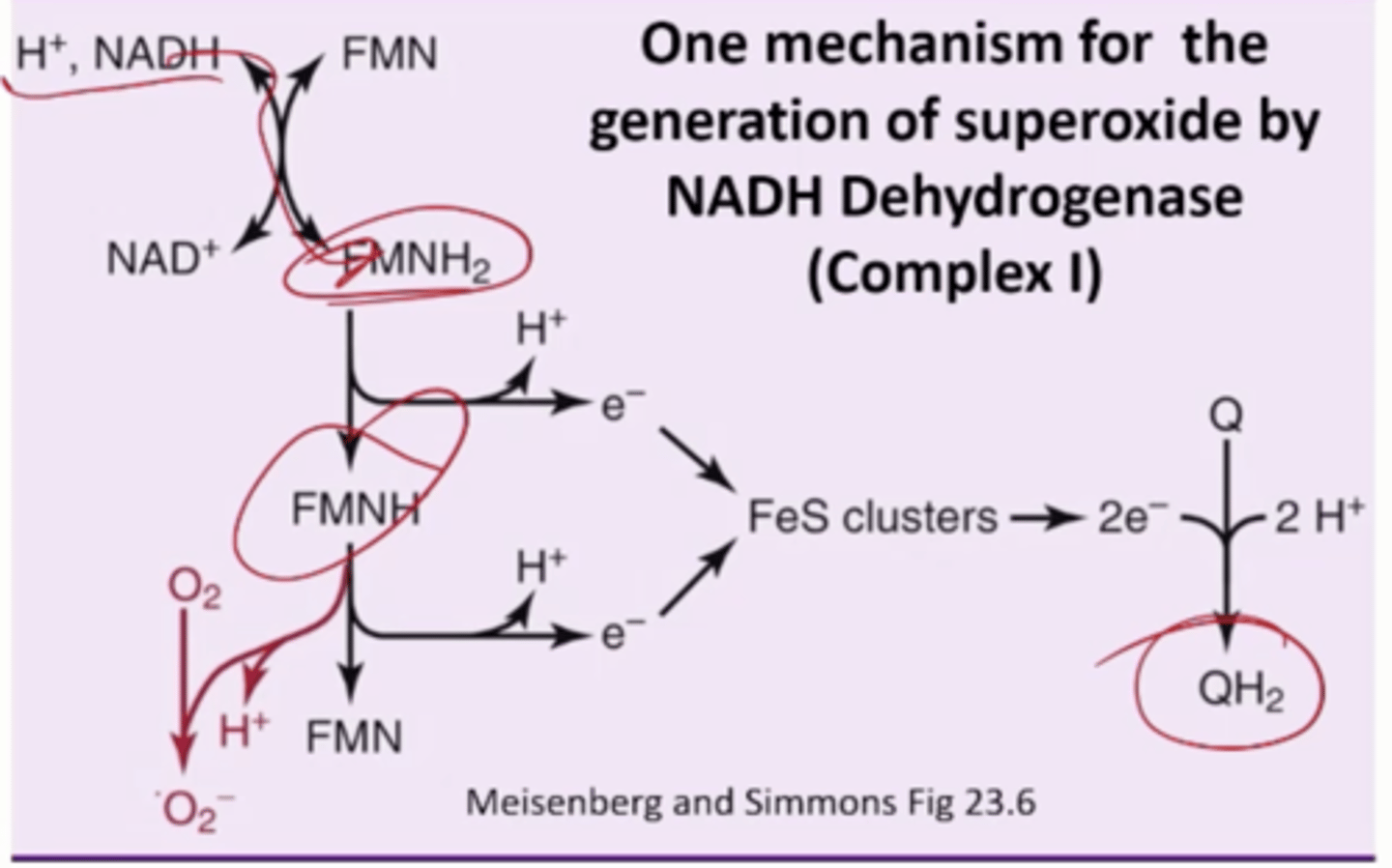
What is one mechanism for the generation of superoxide
NADH Dehydrogenase, Complex 1
NADH Dehydrogenase, Complex 1
NADH. drops off its e- to FMNH2
Transfer e- to waste station, QH2 (1 at a time)
in between
FMNH radical
Free O2 will pick it up
Turn it into superoxide anion
What does free Iron catalyze
Hydroxyl Radical Formation: OH*
iron reduction by superoxide
Superoxide anion can reaction with ferric iron to form oxygen and ferrous iron
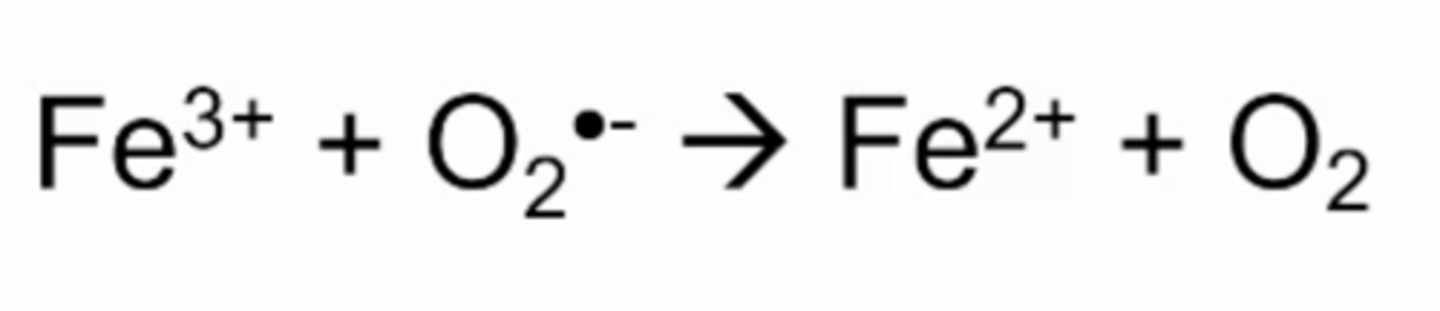
Fenton Reaction
Ferrous iron from superoxide reduction can do another reaction with H2O2
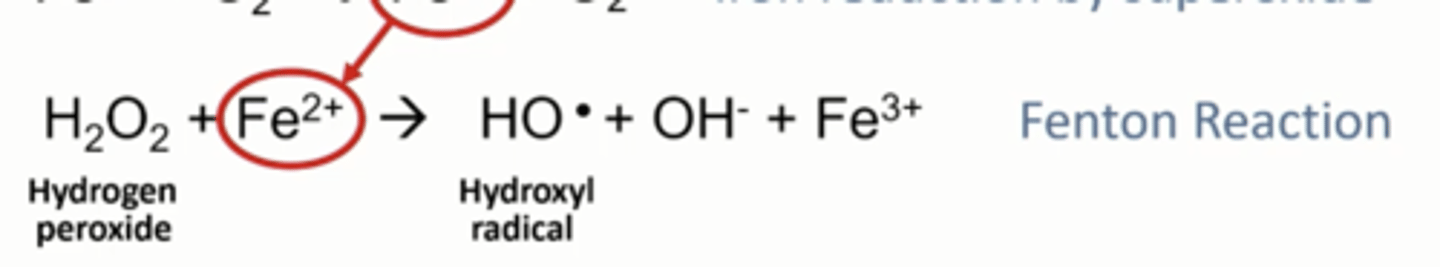
What does the fenton reaction produce
Hydroxyl radical: HO* and regenerates ferric iron
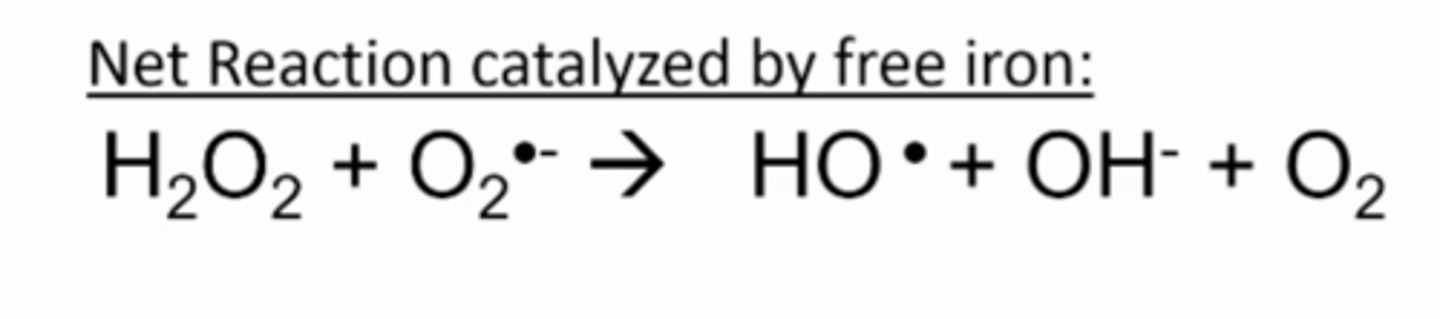
Haber-Weiss Rxn
Iron catalysis of hydroxyl radical formation from superoxide
Where is iron present in the body
Hemoglobin
ETC
Fe3+
Ferric form: most oxidized
Fe2+
Ferrous form: less oxidized
Example: Free radical oxidants (can form free radicals and other highly reactive products)
RO2* Peroxyl
RO- * Alkoxyl
H02* Hydroperoxyl
NO* Nitric oxide
Free radical damages: Proteins, where do they act
On free cystines, oxidation of sulfhydryl group on cys and Met side chains , adds a hydroxyl group, oxidizing it further
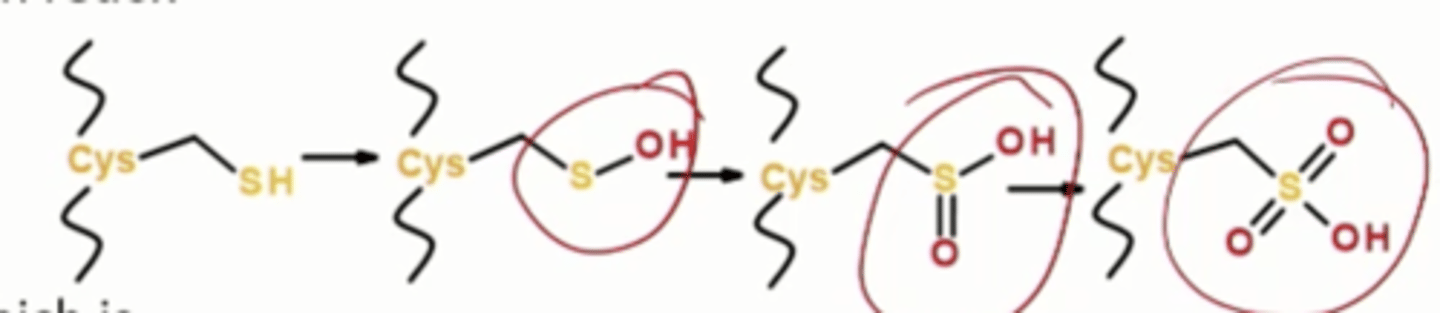
Free radical damages: Proteins, what do they disrupt
Iron sulfur centers in redox enzymes, breakage of peptide bonds
Free radical damages: DNA: Oxidation of bases
8-oxoguanine removed by base excision repair, double-strand breaks
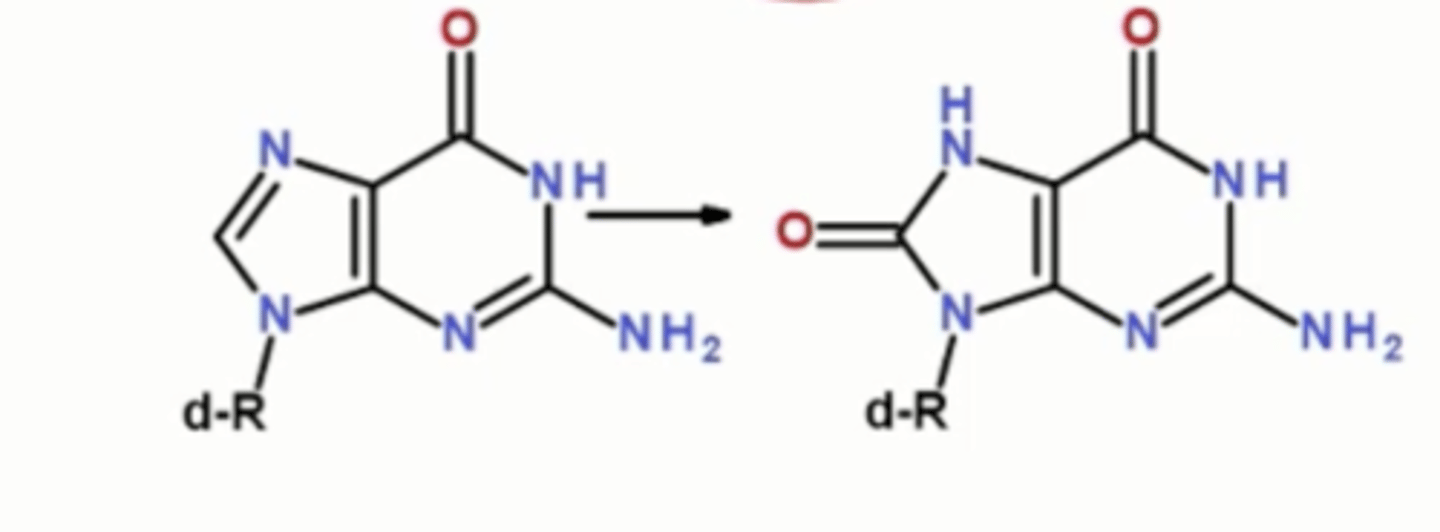
Free radical damages: Lipids
Oxidation of polyunsaturated (not saturated or monounsaturated) fatty acids, causing rancidity
Free radical damages: Lipids Mechanism is what
Free-radical propagation
rancidity
Spoilage caused by breakdown of fats
Lipid Peroxidation: Malondialdehyde formation
Superoxide anion can come in
Polyunsaturated fat can hit C in between
Generates free radical
Cause bond breakage and oxidation
Generate Malondialdehyde
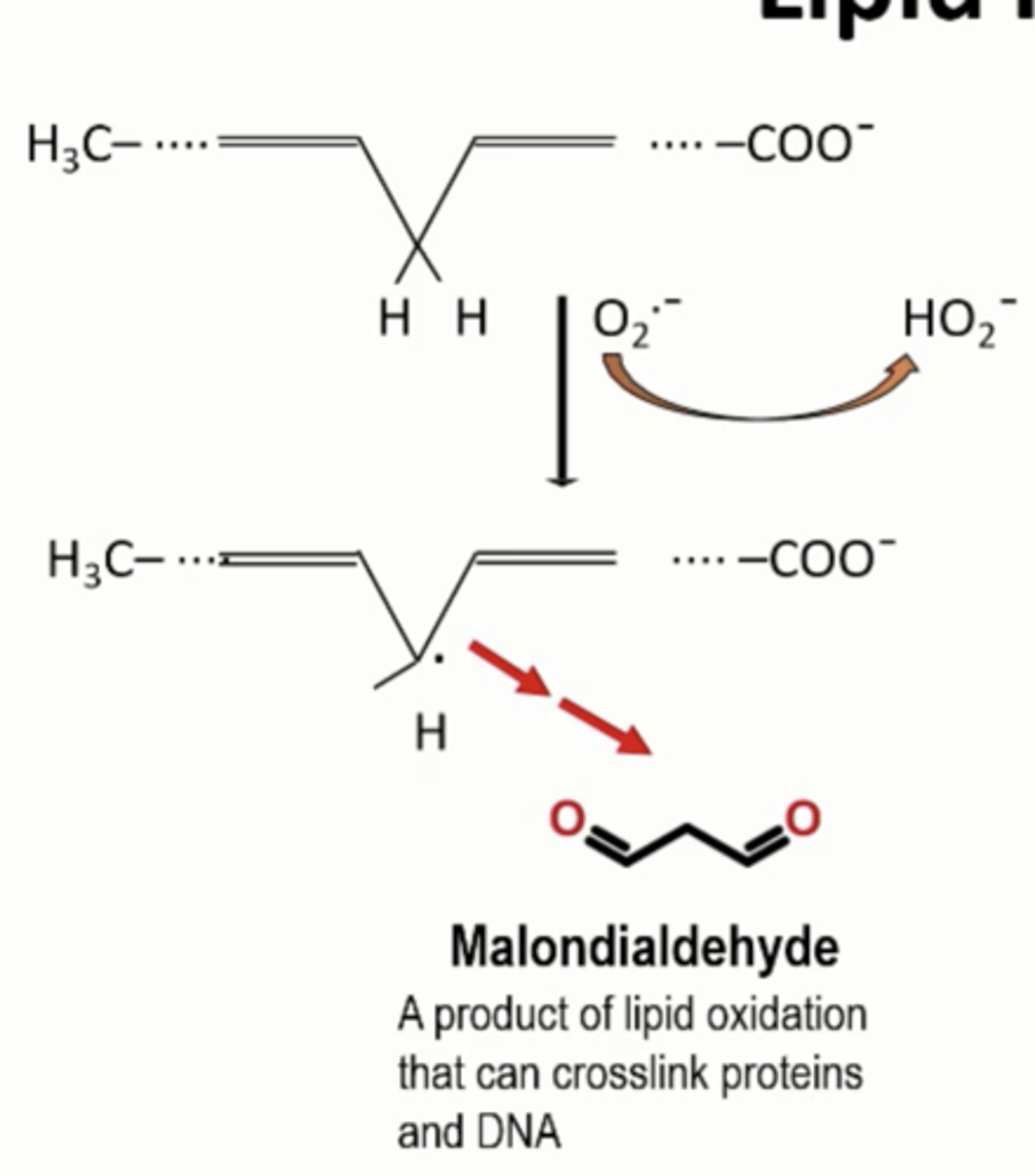
Malondialdehyde
Product of lipid oxidation that can crosslink proteins and DNA, highly reactive and destroy DNA function
Lipid Peroxidation: Free Radical Propagation Mechanism
Single free radical can cause multiple oxidations
Start with OH*
Generate radical: Lipid radical
Free O2 comes in
Modifies and forms peroxide: Lipid peroxyl radical
Transfers free e- to another lipid
Generates Lipid peroxide
Regenerating Lipid radical: Propagation
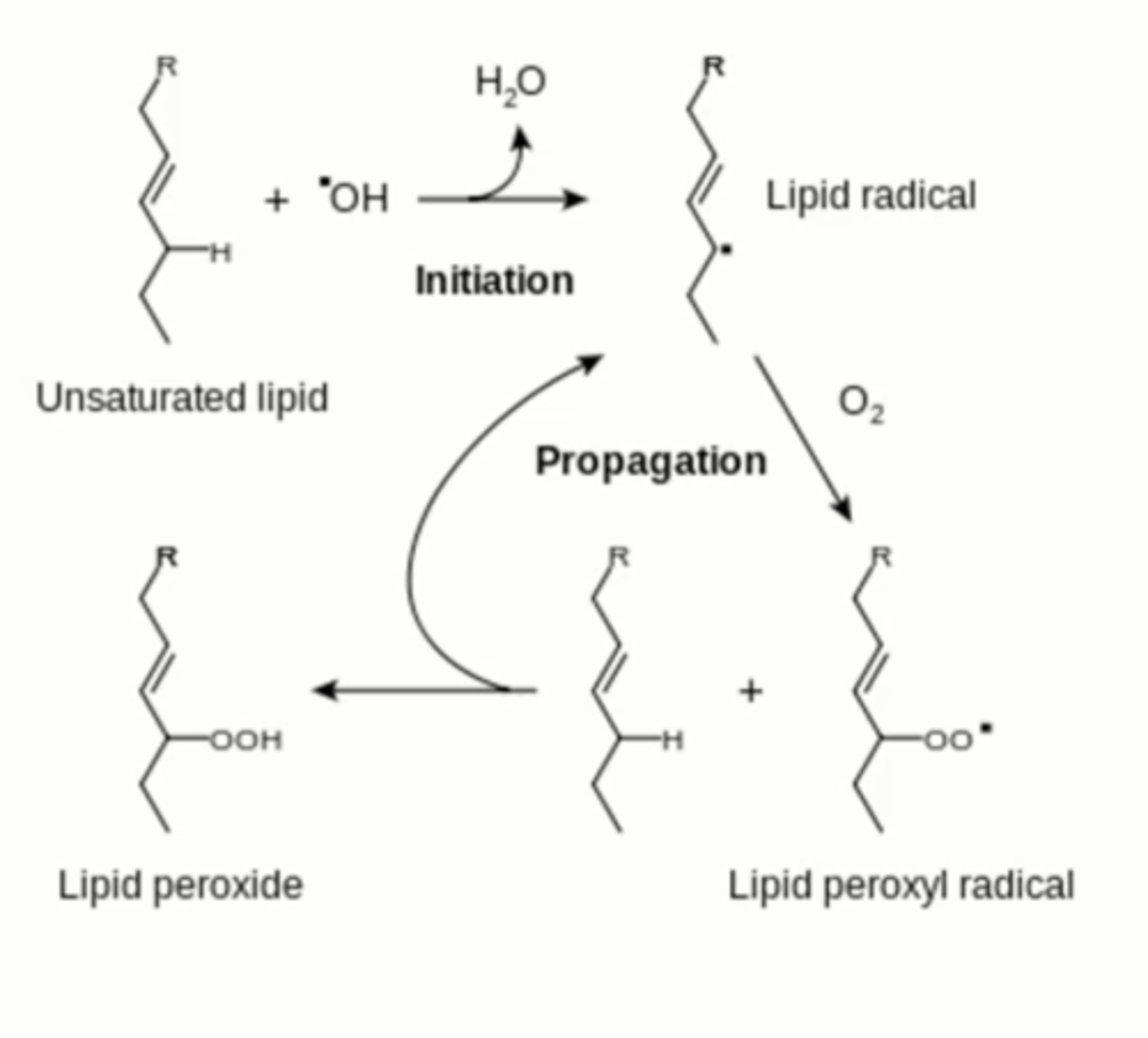
What does Lipid peroxide end up doing
Smelling bad, tasing bad, causing further breakdown of fatty acid chains
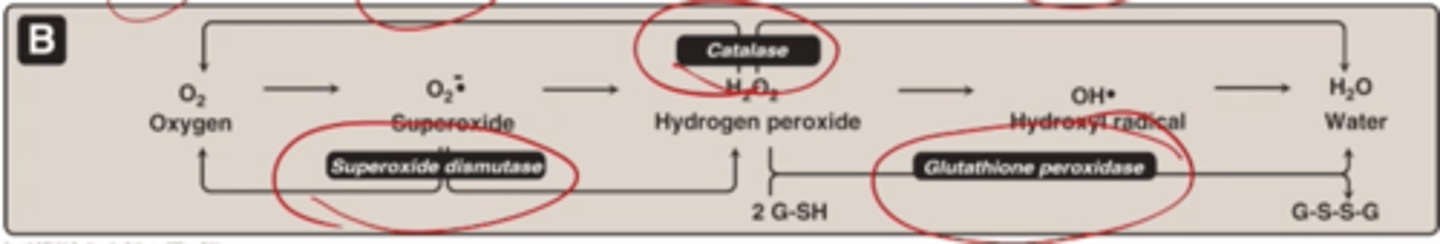
superoxide dismutase
catalyzes conversion of superoxide radicals to molecular oxygen and hydrogen peroxide
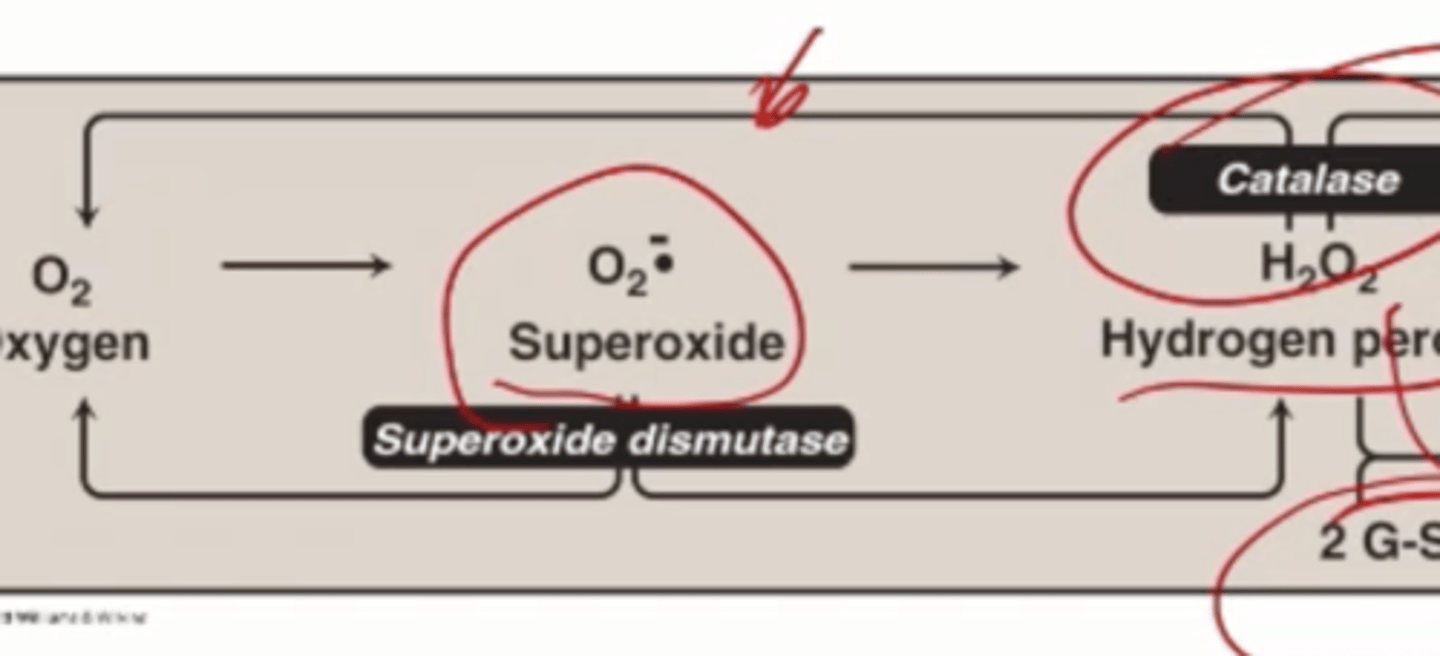
Catalase
breaks down hydrogen peroxide to molecular oxygen water
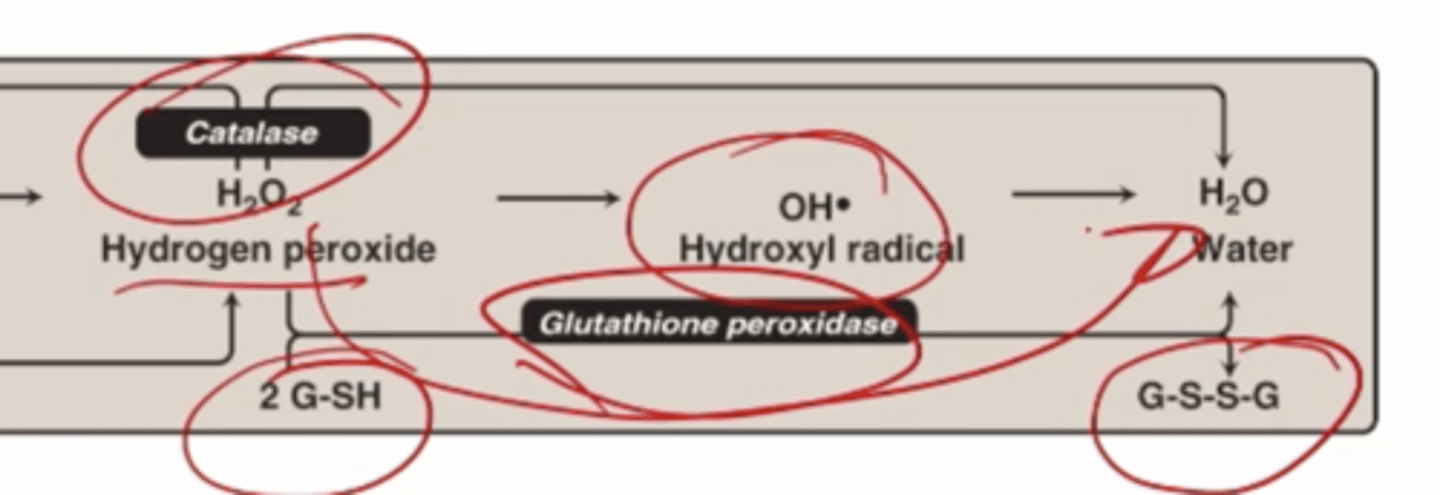
How many H2O2 are needed to generate 1 oxygen and 2 waters
2
Glutathione peroxidase
Can break down H2O2, uses 2 small molecules glutathione in a reduced form (2 G-SH) oxidizes to G-S-S-G and turns H2O2 into water
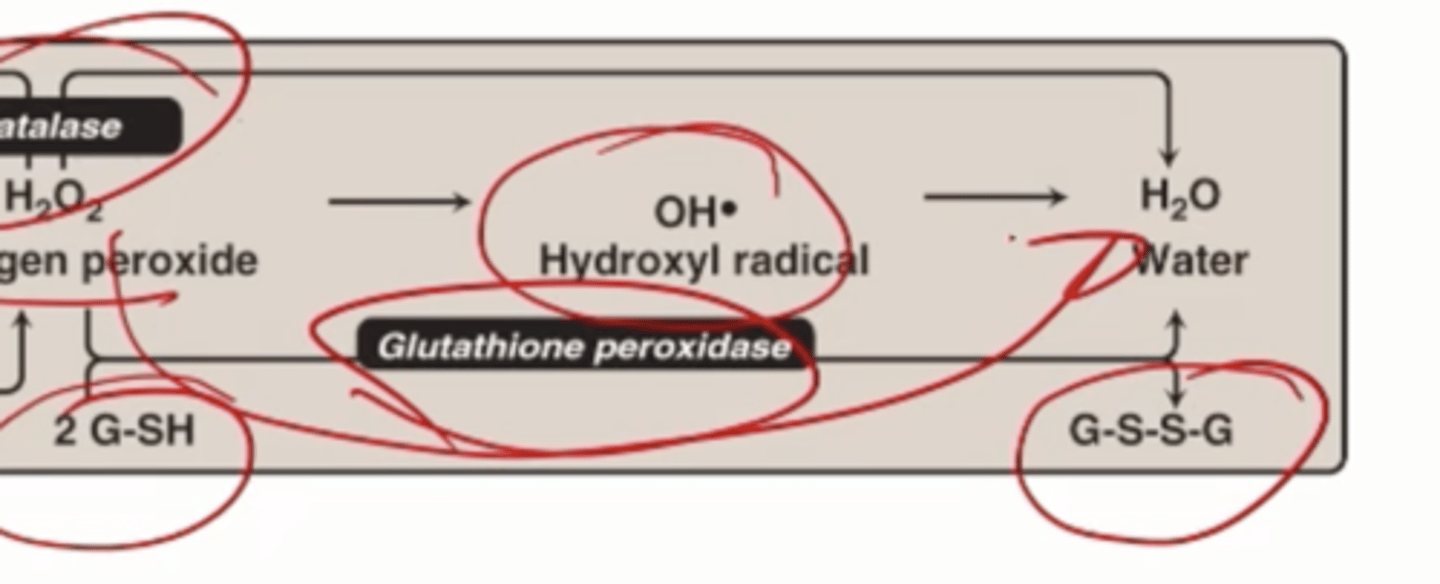
Glutathione is synthesized where
In all cells
Glutathione reduced is what
G-SH
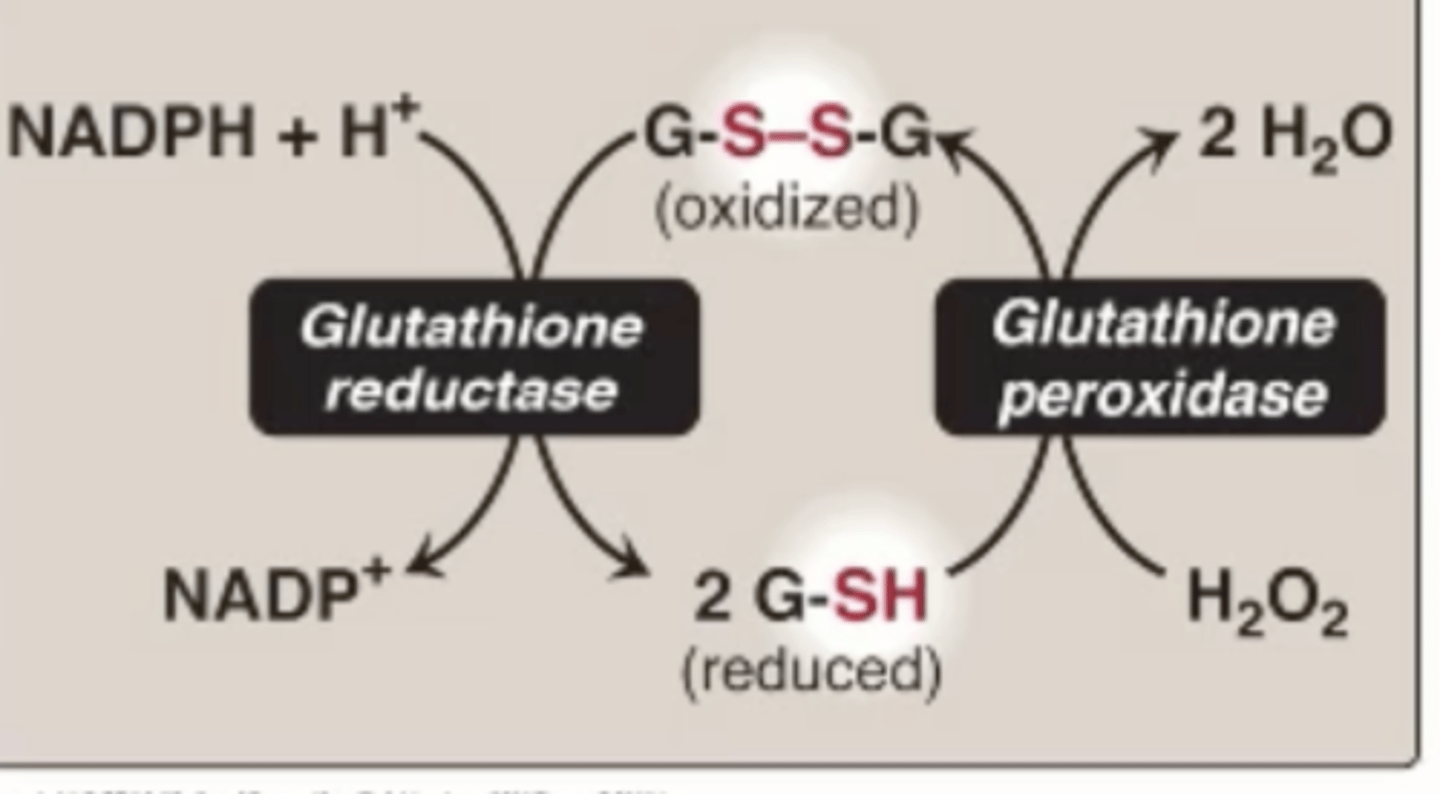
Glutathione oxidized form is what
GS-SG
GS-SG disulfide bonds between cysteines
Chemically the same as disulfides in proteins
Glutathione serves to do what
Maintain cellular redox state
Where is the reducing environment: [GSH] > [GSSG]
Cytoplasm
GSH is regenerated by what
GSSG using NADPH reducing equivalents
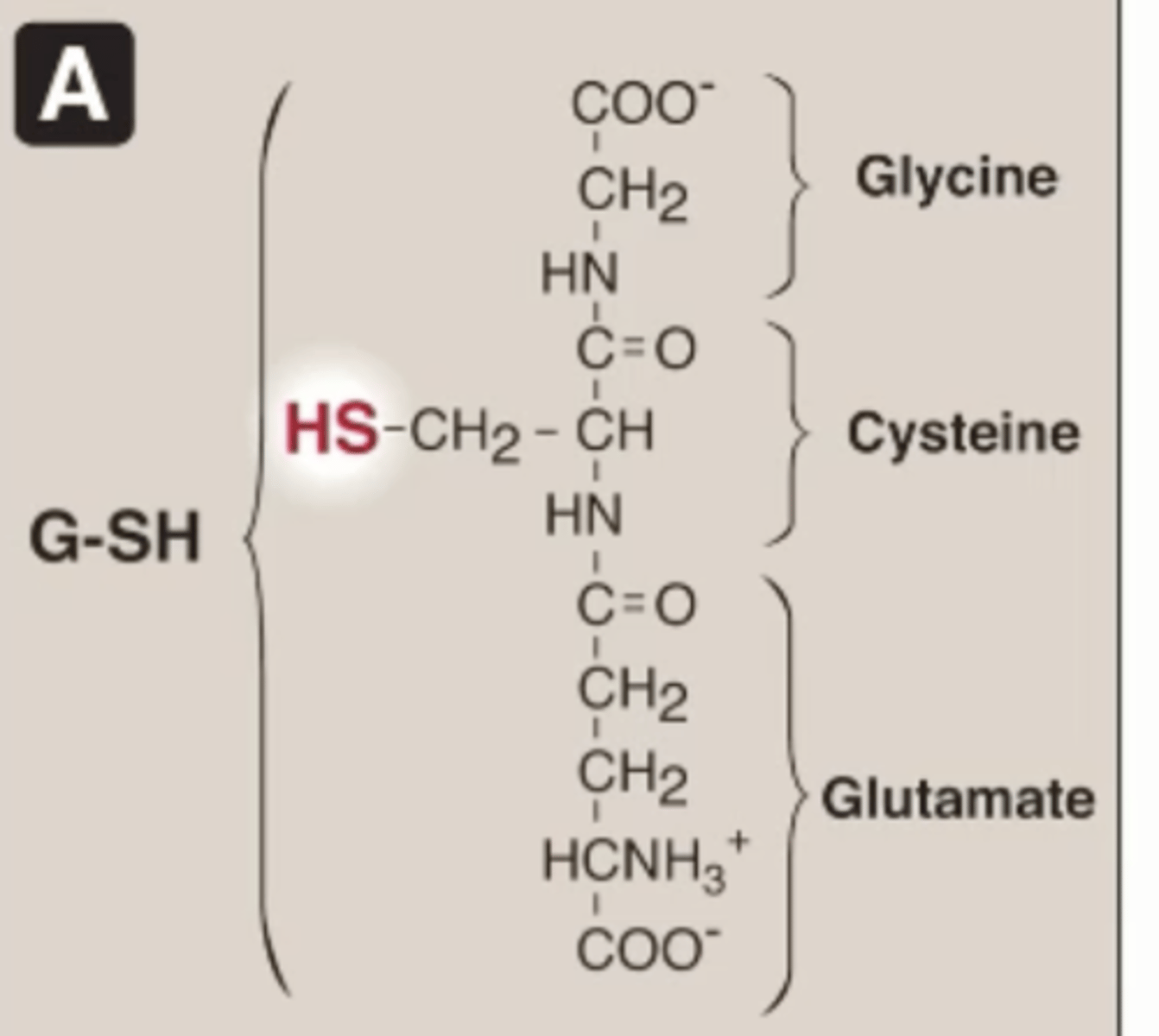
Glutathione structure
Made of 3 amino acids
Not a peptide
Has SH from cysteine
Cytoplasmic NADPH/NADP+
Some irreversible reactions in the metabolic pathways reduce NADP+ to NADPH
How do cells maintain a high NADPH/NADP+ ratio
1. Glucose-phosphate-dehydrogenase (HMP OR PPP)
2. Malic enzyme (Malate to pyruvate)
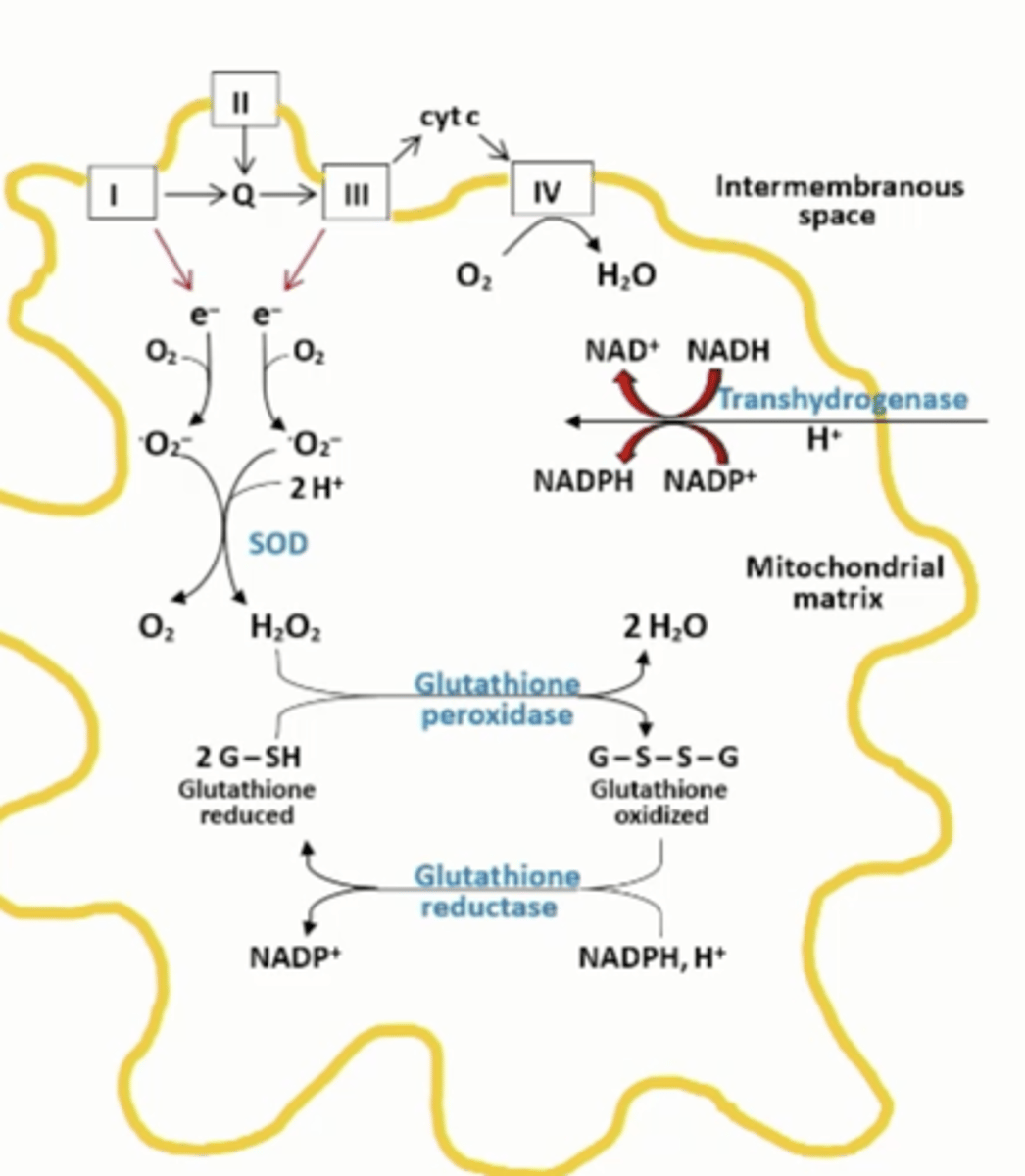
Mitochondrial antioxidant defenses
transhydrogenase that is fueled by the proton gradient uses NADH to maintain a high NADPH/NADP+ ratio for the glutathione reductase reaction
G6PDH deficiency,
impairs the ability of an erythrocyte form NADPH, resulting in hemolysis
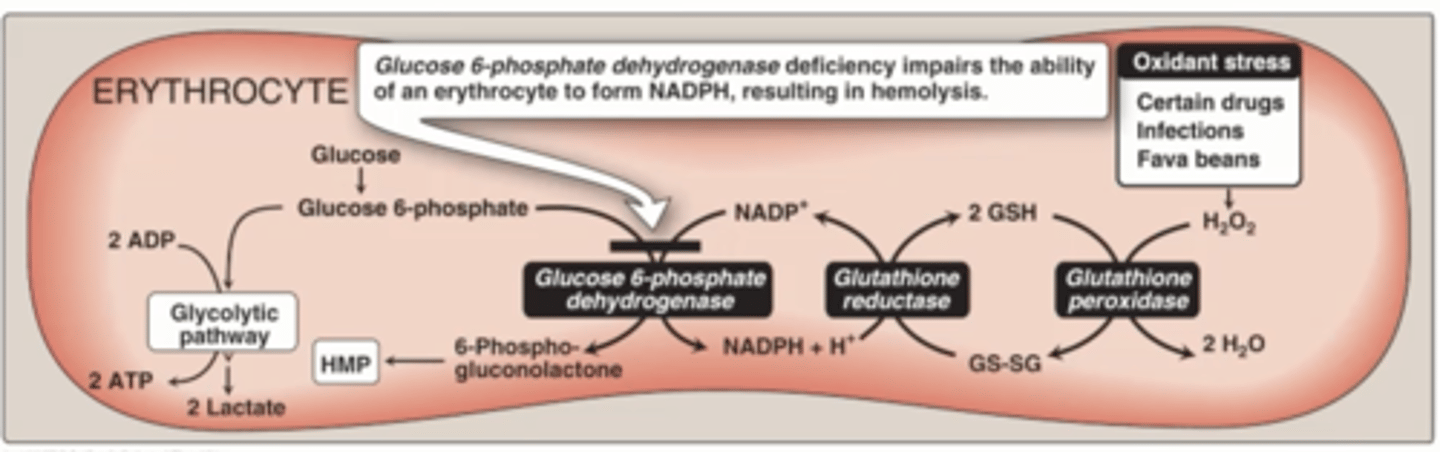
Clinical Correlation: Favism, is what
deficiency in the first enzyme in the hexose monophosphate shunt G6PDH
hemolysis
the rupture or destruction of red blood cells.
Favism is what type of defect
Partial, reduced enzymatic energy
Favism is RBCs can be from what
Oxidant stress
Fava beans
Certain drugs
What happens with oxidant stress in RBCs
Produce more H2O2
Glutathione peroxidase goes into action
Generate GS-SG
Need to regenerates, but not enough NADPH End up in hemolytic crisis in RBCs
400 million have this mutation
Clinical Correlation: ALS Amyotrophic Lateral Sclerosis
Neurodegenerative disorder, late onset with neural degeneration, loss of motor function and associated central neurons
Mutations in what is responsible for ALS
Mutations in superoxide dismutase
ALS results in what
Paralysis, fatal in 2-5 years
20% of familial ALS cases are associated with what
SOD 1 mutations (1-2% of all ALS cases are)
ALS is also known as what
Lou Gherig's disease
Main dietary antioxidants humans use
Ascorbic acid - Vitamin C
What is Vitamin C
natural antioxidant, high requirement of it, co factor is some enzymes (proyl hydroxylase)
Dietary antioxidants: Water-soluble
Ascorbic Acid - Vitamin C
Uric acid
Sulfhydryl compounds
Phytochemicals
Dietary antioxidants: Fat-soluble
Alpha-tocopherol (Vitamin E)
Retinoids (Vitamin A)
Carotenoids (Vitamin A)
Ubiquinone (CoQ, reduced)
Epidemiology
Diets high in antioxidants are associated with health benefits
intervention studies
No consistent benefits when a single antioxidant is given in medium to high doses
Antioxidants
Organic molecules that help protect the body from harmful chemicals called free radicals
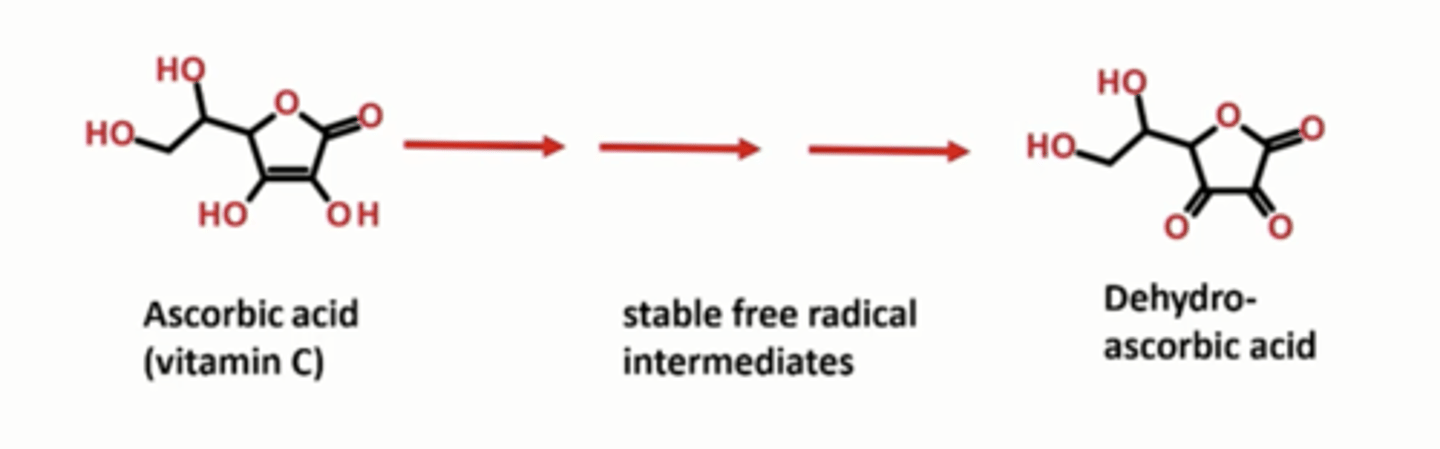
Example: Antioxidants - Vitamin C
Vitamin C can take up e-
Form intermediate free radicals (stable)
Can take up several electrons
End as a stable product that can be excreted in the form of dehydroascorbic-acid
Example Role of NOX2: NADPH oxidase Phagocytosis
Immune system reacted to bacterial invasion
Binds to bacterium
Forms a phagosome, taking in bacterium
NADPH oxidase combines with oxygen, uses NADPH to form superoxide anions
Directly attack bacterium, destroy function of proteins, DNA, and lipids
OR
Can become H2O2 by superoxide dismutase
Myeloperoxidase
Generates hydrochloric acid
Generates hydroxyl radicals
Destroying bacteria
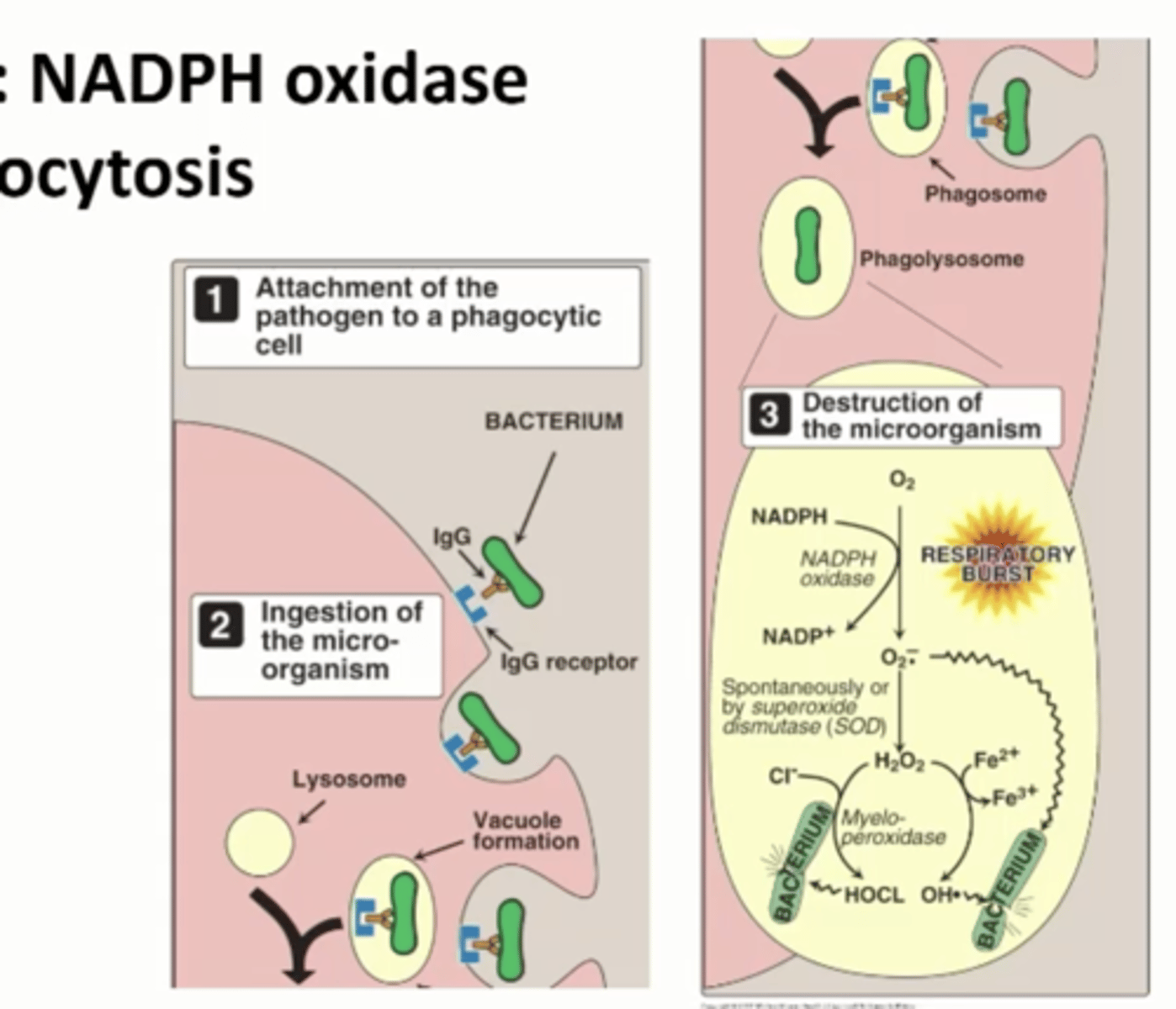
NADPH oxidase Phagocytosis does what
Generate free radicals on purpose during Phagocytosis in order to destroy ingested microorganisms that we want to get rid of
Response system to oxidative stress
Goes through a transcriptional regulatory mechanism
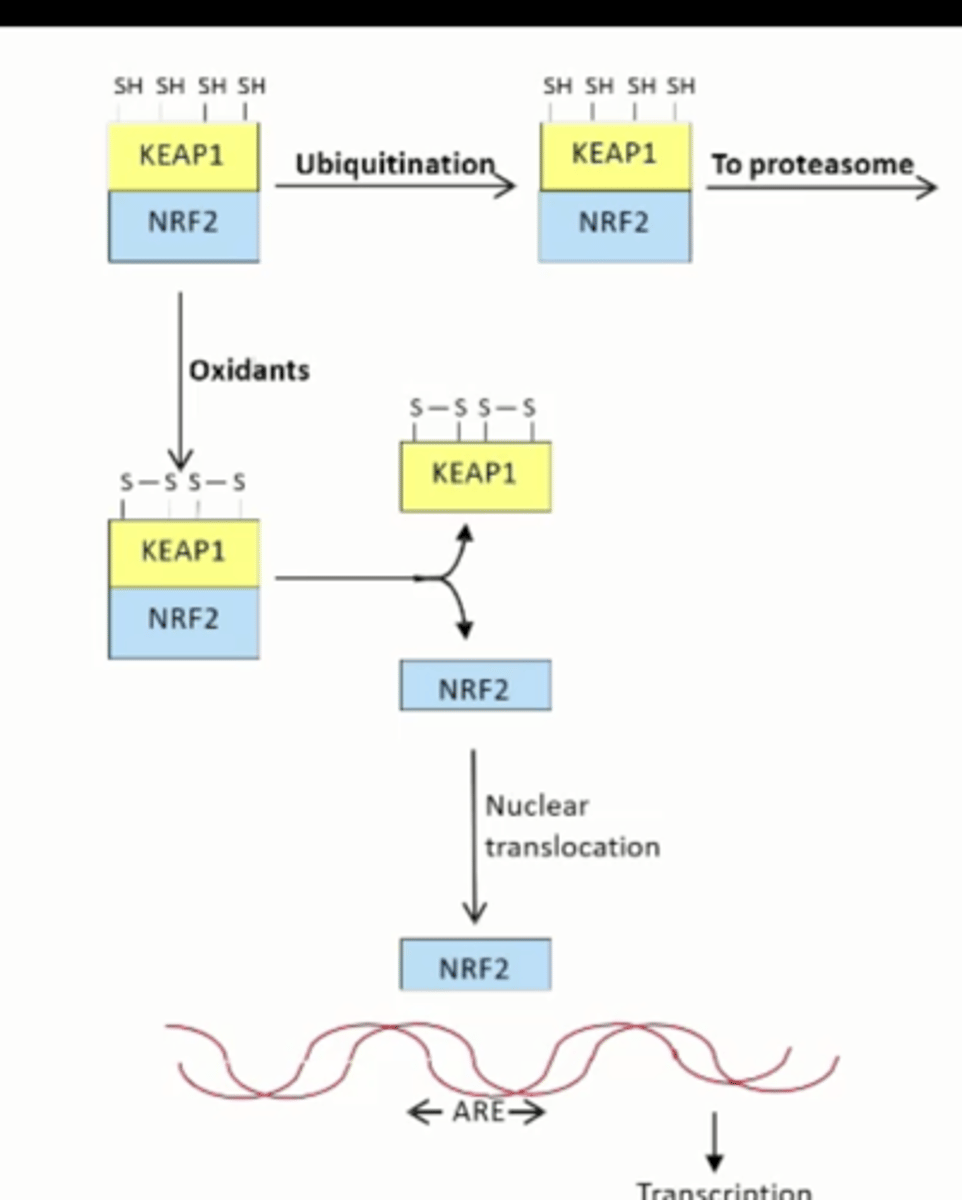
Antioxidant Defense System steps
Have a lot of SH groups
oxidants
Forms disulfides and stabilizes complex
KEAP1 and NRF2 separate
NRF2 goes into the nucleus
OR
No oxidation
SH groups
Ubiquitination
Takes it to the proteasome for degradation
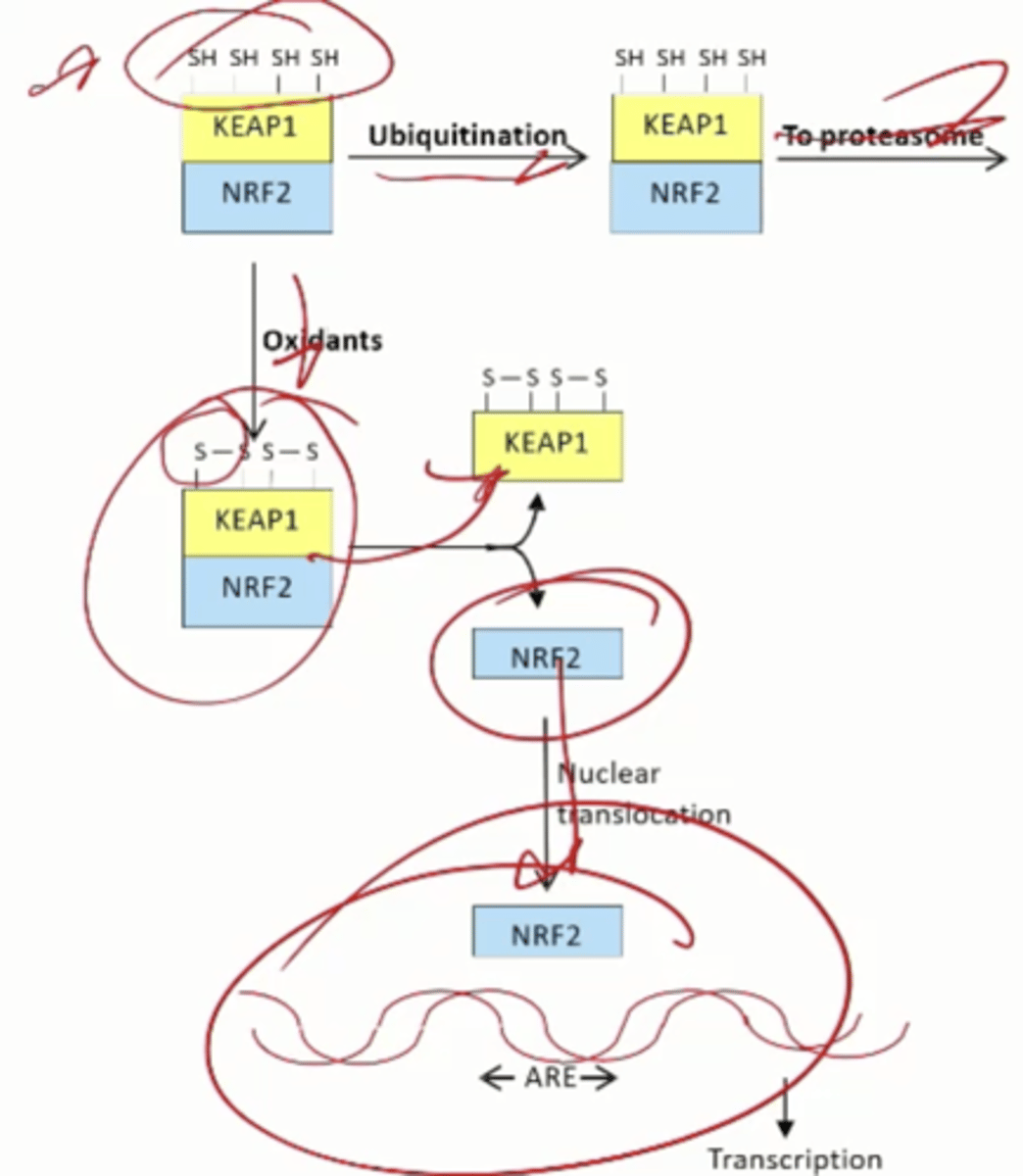
TF NRF2 stimulates what
Transcription of antioxidant genes by binding to the antioxidant response element (ARE) in promoters and enhancers
What happens in the absence of oxidant stres
Kept in the cytoplasm and sent to the proteasome by KEAP1
Oxidants release KEAP1 from NRF2, allowing NRF2 to go to the nucleus and stimulate transcription
Regulated genes
Genes for enzymes of glutathione metabolism and NADPH production, genes for proteostasis proteins (proteasome, autophagy), genes for enzymes of phase 2 drug metabolism
What are 3 words for oxygen deprivation with serious consequences
Hypoxia
Anoxia
Ischemia
What regulates the levels of ROS
Antioxidants and the cellular ROS enzymes
SOD, Catalase, Glutathione peroxidase
Example: Non-radical oxidants
O3 Ozone
1O2 Singlet Oxygen
HOCI- Hypochlorous acid
ONOO- Peroxynitrite