CHEMISTRY AQA PAPER1 gcse
1/131
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
132 Terms
relative charge of a neutron
0
Relative mass of a neutron
1
whats an isotope
Different forms of the same element; same number of protons but different number of electrons
Where is (most of the) the mass of an atom
Nucleus
What is a compound
A substance formed from two or more different elements in fixed proportions, held together by chemical bonds
What is a mixture
two or more elements or compounds that are combined but not chemically bonded together
Explain how paper chromatography could separate a mixture containing three separate dyes
put a dot of each dye mixture on the pencil line and place filter paper in solvent.
The solvent should be below the pencil line
Each different dye will move up the paper at a different rate, separating them
When do u use filtration
On a mixture of an insoluble solid and aliquid
When to use crystallisation
To separate a soluble solid from a solution
How does filtration work
Passing the mixture through a filter
Insoluble particles caught by the filter
Why crystallisation insead of evaporation
If the salt will decompose when heated.
Or want big crystals of salt
How to do crystallisation (makins salts practical)
Heat the acid using bunsen burner until almost boiling
Add small amounts of copper oxide powder and stir
Continuously add copper until it is in excess
Transfer remaining solution into crystallising dish and leave in cool place for atleast 24h
Remove the crystals and gently pat dry to remove excess liquid
How to separate sand and salt mixture
Filtration then crystallisation
when to use fractional distilation
Mixtures of liquids including those of similar boiling points
When to use simple distillation
To separate a liquid from a solution
Diff boiling points
How to fractional distillation
Flask below a fractionating column Connected to a condenser and has a thermometer at the top
Pour mixture into flask and heat to bliling point of liquid you want to separate out
Desired liquid should evaporate and travel up fractionating column and into the condenser
In condenser it turns back into a liquid where it can be collected.
Plum pudding model
Atoms as balls of positive charge with electrons stuck to them
Bohr arrangement
Electrons orbit the nucleus in shells that are a fixed fistance from the nucleus
How were alpha particles used to disprove plum pudding model
Fired at a thin sheet of gold. Most particles went straght through the sheet but some were deflected
This meant the positive charge couldnt be spread out evenly between the atoms, as the plum pudding model described
electronic structure
max 2 first shell
max 8 in other shells
Elements in the same group (column)
Have the same number of electrons in their outer shell
What is a metal
Elements which can form positive ions
Why can elements toward the bottom of the periodic table lose electrons easily
More shells of electrons than elements at the top, so outer electrons are further from the nucleus - weaker attraction
Where r metals on the periodic table
Toward left and bottom
Where transition metals on Ptable
Centre
Metals properties
Strong, malleable, good conductors of elec, high melting and boiling points
Transition metals compared to group 1 metals
More dense than group 1
Higher melting points
iron in the harber process
A catalyst to speed up the reaction
(Transition metals make good catalysts)
Alkali metals (group 1 metals)
reactivity increases as you go down the column. Becuase the outer electron is further away from nucleus so less attracted and more easily lost.
Potassium with water
Vigorously
Hydrogen produced so bubbling and fizzing
Flame
Alkali metals properties
Soft, low density
Group 7 (halogen ions) charge
Need to gain one electron to complete outer shell, form 1- ions
What happens as you go down group 7 in Ptable
Boiling points and melting points of the halogens increase
Trend of reactivity decreases becuase its harder to gain an electron as there is thess attraction as the outer shell becomes further from the nucleus
Halogen molecules
All halogens exist as molecules such as f2
Group 0 elements
Noble gases
Nobles gasses monatomic gases
Are single gases not bonded to each other
Noble gases inert
because they have full outer shells so dont need to lose or gain any electrons to become stable
Metal + oxygen
Metal oxide
Who discovered neutrons
Chadwick
Mass number
Protons + neutrons
Atomic number
Num of protons and num of electrons
Metal + water
Metal hydroxide
Solid
Fixed state cant flow but can vibrate
Cant be compresed
Liquid
Can move
Not in a fixed position
Can flow
Cant be compressed
Gas
Mot fixed
Can flow
Can be compressed
Liquid + liquid → solid OBSERVATIONS
Cloudy
Liquid + solid → gas OBSERVATIONS
Bubbles
Loss of mass
Fizzing
Liquid + liquid → gas OBSERVATIONS
Bubbles
Loss of mass
Fizzing
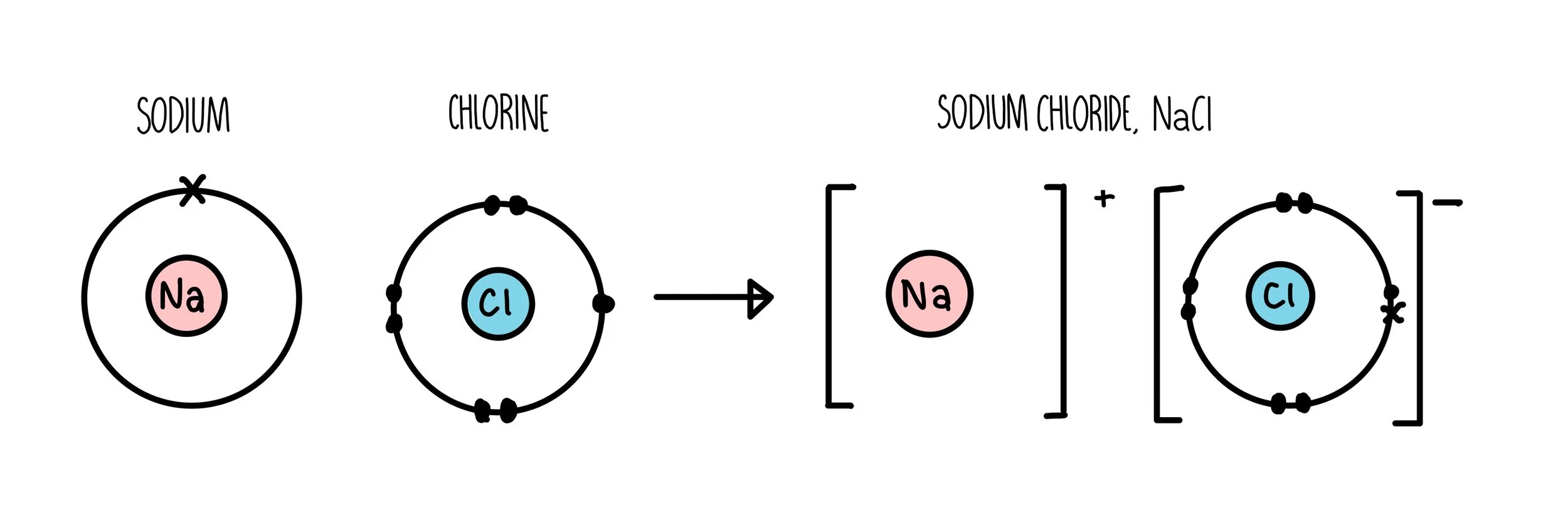
ionic bonding
The transfer of electrons from a metal to a non metal
Group 1 ionic bonding
Forms 1+ ion
Group 2 ionic bonding
Forms 2+ ion
Group 6 ionic bonding
Forms 2- ion
Group 7 ionic bonding
Forms 1- ion
Ionic bond
The electrostatic Attraction between oppositely charged ions
Ionic bonding diagram
Covalent bonding
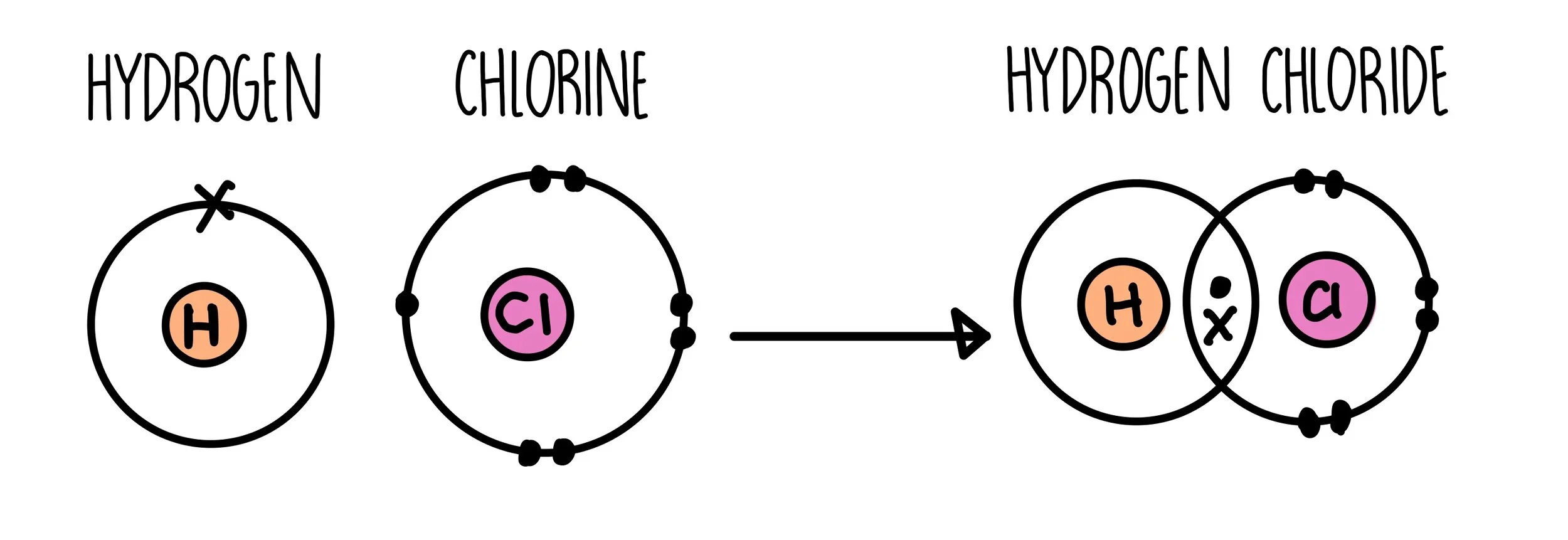
The sharing of electrons between two non metals
Covalent bonding diagram
melting point of ionic compounds
High melting point
Because it takes alot of energy to overcome the strong electrostatic attraction between the oppositiely charged ions
Electrical conductivity of ionic compounds
Good electrical conductivity when liquid or aqueous NOT when solid
Because when liquid or aqueous there are charged particles that can move and carry charge throughout the structure
Dissolve? Ionic compounds
sometimes/often dissolve
Depends if the attraction between the ions and water is greater than the attraction between the ions
Ionic compounds strength?
Strong but brittle
Electroststic repulsion shatters the lattice due to strong bonds (if one layer is moved)
Giant ionic lattice
Positively and negatively charged ions attract each other in all directions to form this
(One ion is ionically bonded to many others)
2 types of Covalent structures
Simple molecular
Giant lattice
Covalent simple molecular boiling point
Relatively low melting points
Doesnt take much energy to overcome the weak intermolecular forces
Covalent simple molecular electrical conductivity
Does not conduct electricity
There are no charged particles to move or carry charge throughout the structure
Covalent simple molecular dissolve?
Does not dissolve in water but does in organic solvents
Polymers
A substance made up of many monomers joined together
Diamond structure
Giant covalent lattice
Each carbon atom forms 4 covalent bonds with other carbon atoms
Diamond electrical conductivity
Poor/none because there are no charged particles to move or carry charge throughout the structure
Diamond melting point
High melting/boiling point
it takes alot of energy to overcome the strong covalent bonds
Diamond soft/hard
very hard
The atoms are held in place by strong covalent bonds
Why graphite in lead of pencils
The layers are scraped off onto the paper due to the weak attraction between the carbon atoms with no covalent bonds between the layers
Graphite structure
giant covalent lattice
Each carbon atom forms 3 covalent bonds w/ other carbon atoms, forming layers of hexagonal rings (with no covalent bonds between layers)
One electron from each carbon atom is delocalised
Graphite electrical conductivity
Can conduct electricity
Because the delocalised electrons can move and carry charge throughout the structure
Graphene structure
A single layer of graphite →
One layer of hexagonal rings of C atoms covered in delocalised electrons
Graphene uses
Electronics - becuase it conducts electricity
Composites - because it has a high strength to weight ratio
Fullerenes structure
Hollow cage of C atoms based on graphene
Hexagonal rings of C atoms although may also contain 5C or 7C rings
Very long and thin → high length to diameter ratio
Diameter = 1 × 10^-9m
Buckminster fullerenes (bucky balls) structure
Carbon nanotubes
C60 (60 carbon atoms all together)
Simple molecular
Bucky balls uses
Lubricant or reinforcement
Metalic bonding structures
Giant metallic lattice
Ionic bonding structures
Giant ionic lattice
Metallic bonding structures
Giant metallic lattice
Giant metallic latice structure
Metal ions close packed
Delocalised electrons in the structure
metals melting point
High melting point
It takes alot of energy to overcome the strong electrostatic attraction between the positively charged ions and the delocalised electrons
Metals malleable?
Yes
The layers of ions can slide over each other as they are in a sea of delocalised electrons and are not in a fixed position - soft
Metals electrical conductivity?
Good electrical conductivity
Because the delocalised electrons can move and carry charge throughout the structure
Metals therman conductivity?
Good thermal conductivity
The delocalised electrons can move and transfer heat energy through the structure
Alloys
Mixtures of a metal with atleast ine other element (usually another metal)
Alloy strong?
Stronger and harder than a pure metal becuase the layers cant slide over eachother
Alloys electrical conductors
Less efficient electrica conductors than pure metals
Have irregularities in the lattice → scattered delocalised electrons, so less easy for electricity to carry through the structure
Nanoparticle measurement
1nm = 1 × 10^-9m
nanoparticle SA
high SA to volume ratio → smaller quantities needed to be effective.
Nanoparticle uses
Cosmetics, medicine, electronics, catalysts
Nanoparticle issues
Small enough to go through pores and into blood stream which can cause problems (cosmetics)
Percentage yield
(Actual yield / theoretical yield) x 100
Why can % yield sometimes be under 100
Some products lost on transfer
Could be a redox reaction
Unexpected side reactions
moles
Mass / mr
Atom economy
(Mr of desired products / total mr of reactants) x100
moles to volume
x 24 (24dm³)
volume to moles
divide by 24 (24 dm³)