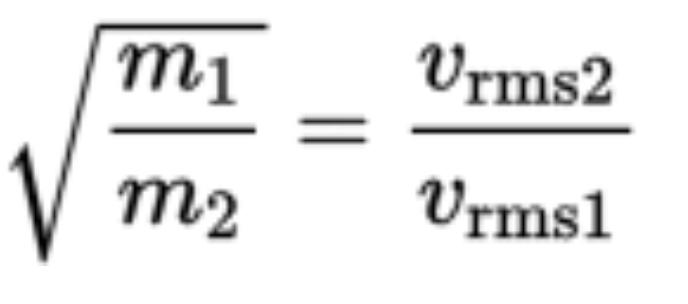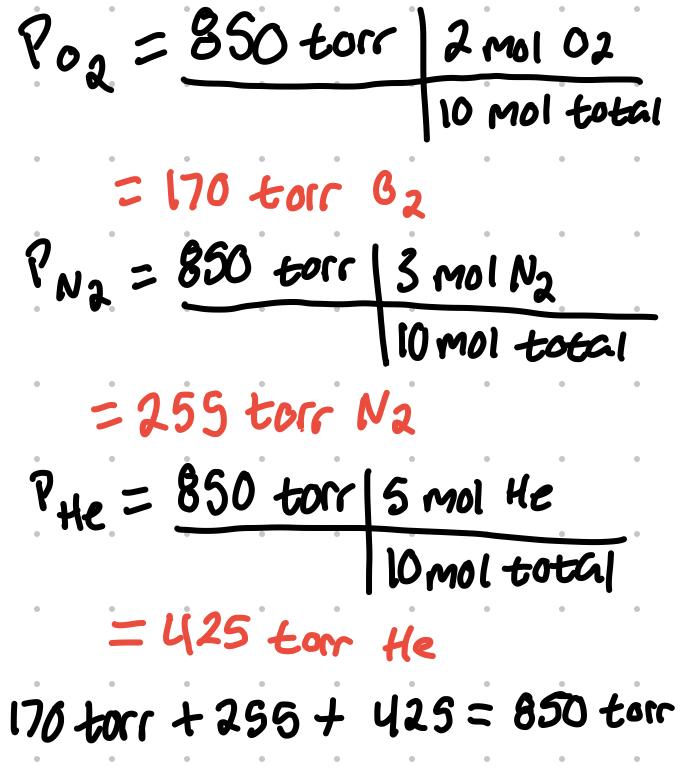Chapter 6: Gasses
- The ideal gas law describes how pressure, volume, temperature, and moles of gas change.
- Kinetic molecular theory describes the behavior of all gasses and why they behave the way they do
The Gas Laws
- Gasses have four properties
- Temperature (T)
- Pressure (P)
- Volume (V)
- Moles of gas (n)
- Each gas law holds two properties constant while one of the properties is changed
Boyle’s Law
- Boyle’s law is the inverse pressure-volume relationship
- If a sample of gas starts with initial conditions of pressure and volume and an experiment is done ONLY affecting pressure and volume, you get the equation
- PiVi=PfVf
- i = initial
- f = final
Charles’s Law
- Charles’s law is the direct relationship of temperature and volume
- (Vi)/(Ti) = (Vf)/(Tf)
- Absolute zero is the lowest possible temperature.
- -273 celsius or 0 Kelvin
Gay-Lussac’s Law
- Gay-Lussac’s law is the direct relationship of pressure and temperature
- (Pi)/(Ti)=(Pf)/(Tf)
Avogadro’s Principle
- Also known as Avogadro’s Law, it describes that equal numbers of molecules/atoms contain equal volumes of gases under identical conditions of temperature and pressure
- (ni)/(Vi)=(nf)/(Vf)
Ideal Gas Law
Combining the previous gas laws, the ideal gas law is obtained
- PV = nRT
- R is the universal gas constant. In this equation, R is .08206 L·atm/mol·K
Example
A gas occupies 250 mL, and its pressure is 550 mmHg at 25°C.
If the gas is expanded to 450 mL, what is the pressure of the gas now?
What temperature is needed to increase the pressure of the gas to exactly 1 atmosphere and 250 mL?
How many moles of gas are in this sample?
The sample is an element and has a mass of 0.525g. What is it?
Standard Temperature and Pressure (STP)
- If a gas is stated to be at STP, it will be at 1 atm and 273 kelvin
Molar Mass, Density, and Molar Volume
- Molar mass can be determined if P, V, g, and T are known
- PV = (g/molar mass)RT
- Density can be determined if P, T, and molar mass are known
- P(molar mass) = (g/V)RT
- Molar volume can be determined by rearranging the ideal gas law equation
- (V/n) = (RT/P) or
- (V/n) = 22.4 L/mol if at STP
Kinetic Molecular Theory
Kinetic molecular theory describes gasses at the particle level.
- Gasses consist of molecules or atoms in continuous random motion
- Collision between molecules/atoms are elastic
- Volume taken up by gaseous molecules is negligibly small
- The attractive and repulsive forces between gaseous molecules is negligible
- Average kinetic energy of gaseous molecules is directly proportional to the Kelvin temperature of the gas
Pressure is determined by the velocity of gas particles colliding with container walls. Changing temperature changes the force of collision in addition to the frequency.
If the volume of a container is decrease, the particles will collide with the wall more frequently, and pressure will increase
By increasing temperature, the average kinetic energy is increase so the particle velocity is increased, and the pressure will increase since the collisions are stronger
Graham’s law of effusion compares the rate of effusion of two gasses and says the rates are inversely related to the square root of the mass of the gas particles
Effusion through a pinhole in a vacuum requires a gas to hit the pinhole just right in order to escape. More collisions mean a higher rate of effusion, or a higher likelihood that it will escape.
Average Kinetic Energies and Velocities
- Average kinetic energy is sometimes higher or lower than estimated.
- KE = (.5)mv^2
Real Gasses
- The ideal gas law does not work well at very high pressures or very low temperatures
- Gases close to the condensation point will deviate slightly because it breaks two gas assumptions: gasses have no volume and have no repulsive/attractive forces
- An ideal gas must follow the assumptions stated earlier.
Dalton’s Law of Partial Pressures
Dalton’s law of partial pressures says that if two gasses are mixed together, they will act independently of each other.
- Total pressure is the sum of all partial pressure of gasses in a container
Example
A mixture of gasses contain 2 mol of O2, 3 mol of N2, and 5 mol of He. Total pressure is 850 torr. What is the partial pressure of each gas?
Experiments Involving Gases
- Pneumatic troughs are used to collect gases produced in a reaction vessel.
- To find the gas collected in pressure,
- Pgas = Patm - Pwater