Unit 2: Chemistry of Life
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
93 Terms
acid
a substance that donates hydrogen ions and therefore lowers pH
adhesion
the attraction between water molecules and molecules of a different substance
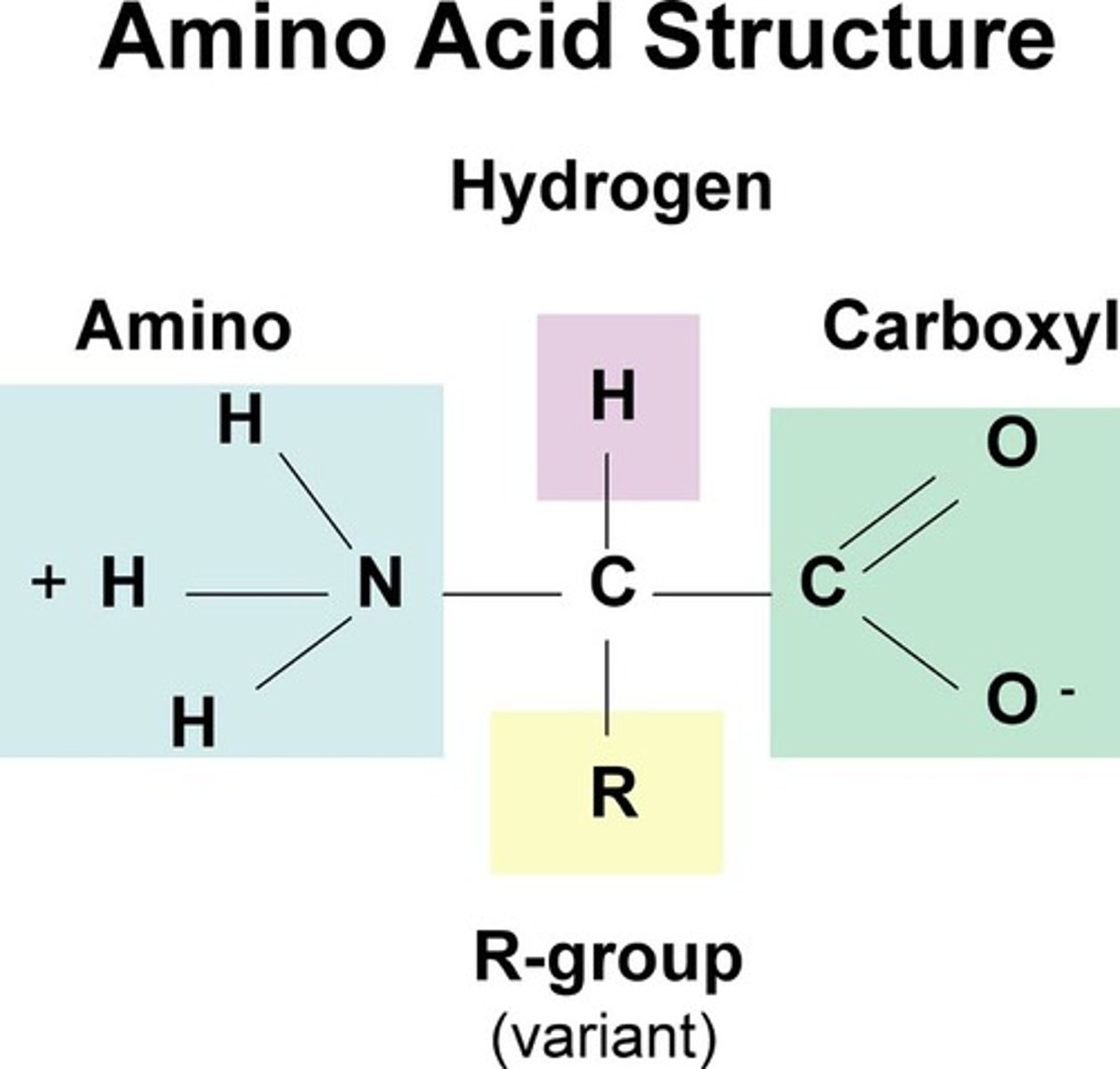
amino acid
a monomer of a protein
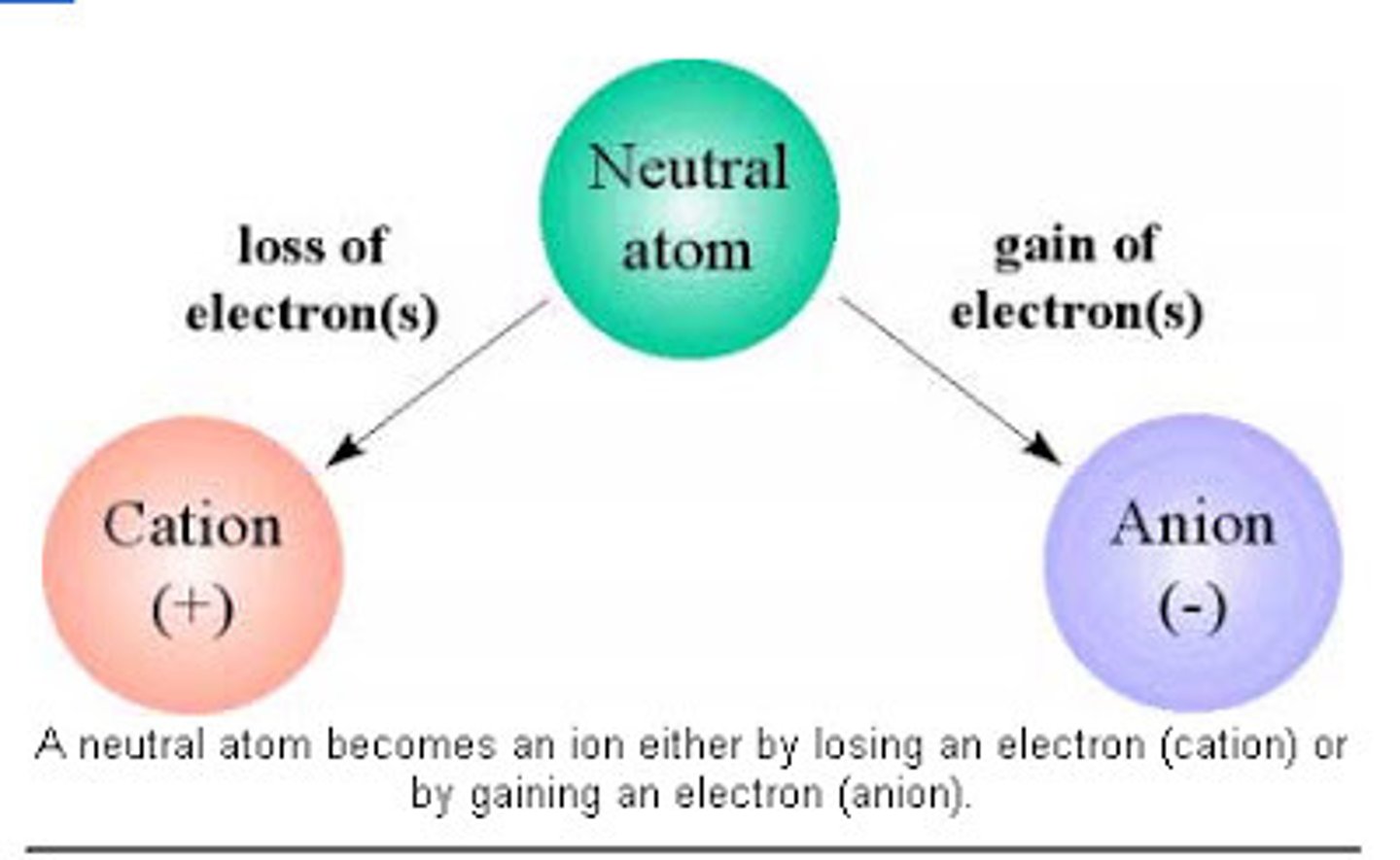
anion
a negative ion formed by gaining electrons
base
a substance that absorbs hydrogen ions and therefore raises pH
buffer
a solution that resists a change in pH by absorbing or releasing hydrogen or hydroxide ions
carbohydrate
a biological macromolecule in which the ratio of carbon to hydrogen to oxygen is 1:2:1; carbohydrates serve as energy sources and structural support in cells
cation
a positive ion formed by losing electrons
cellulose
a polysaccharide that makes up the cell walls of plants and provides structural support to the cell
chemical bond
an interaction between two or more of the same or different elements that results in the formation of molecules
chitin
a type of carbohydrate that forms the outer skeleton of arthropods, such as insects and crustaceans, and the cell walls of fungi

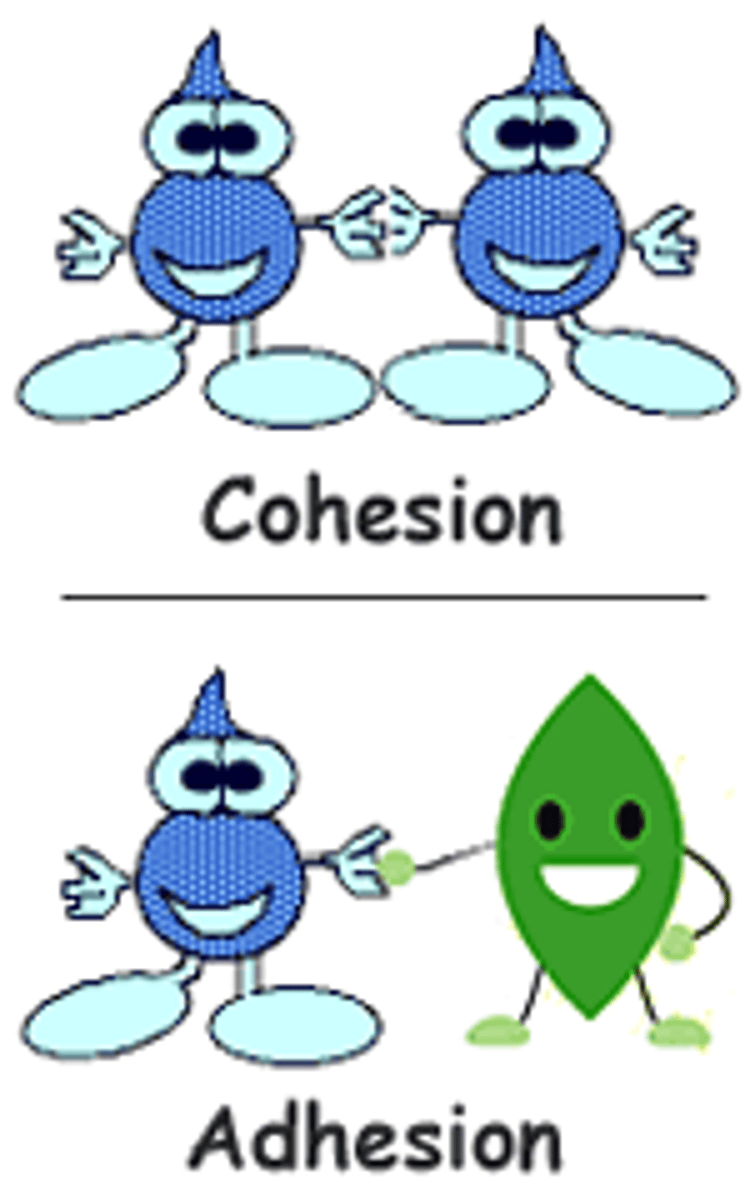
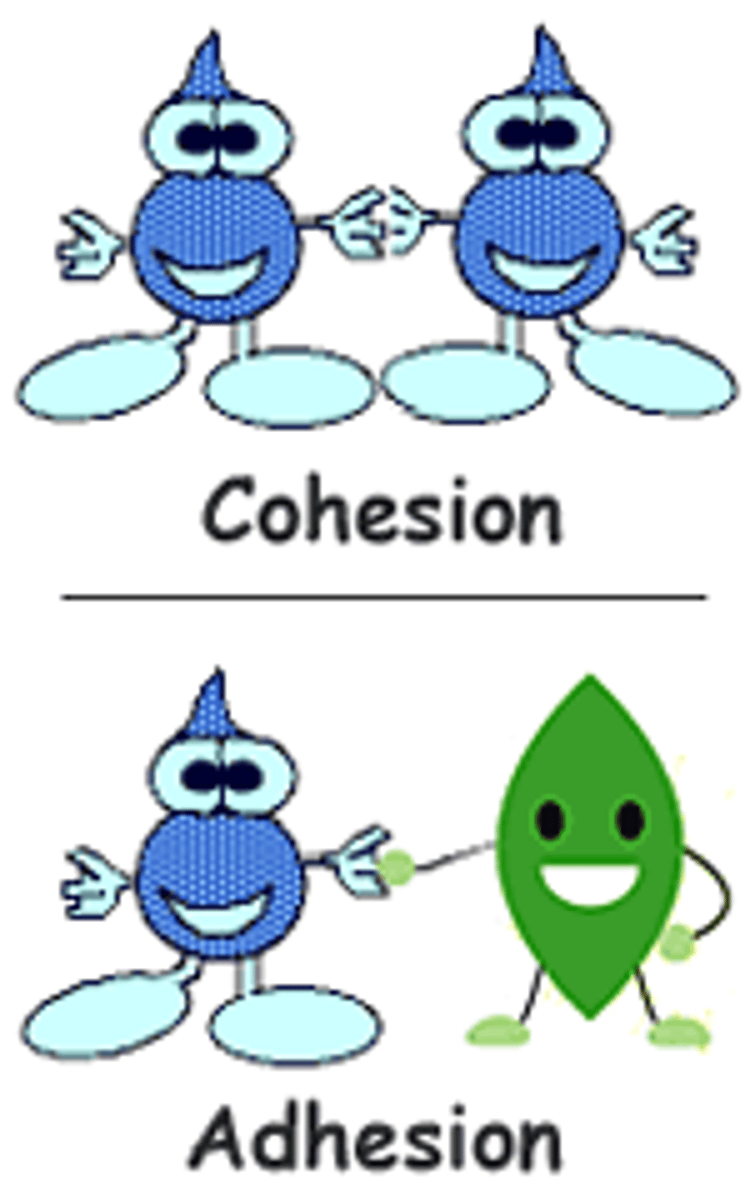
cohesion
the intermolecular forces between water molecules caused by the polar nature of water; creates surface tension
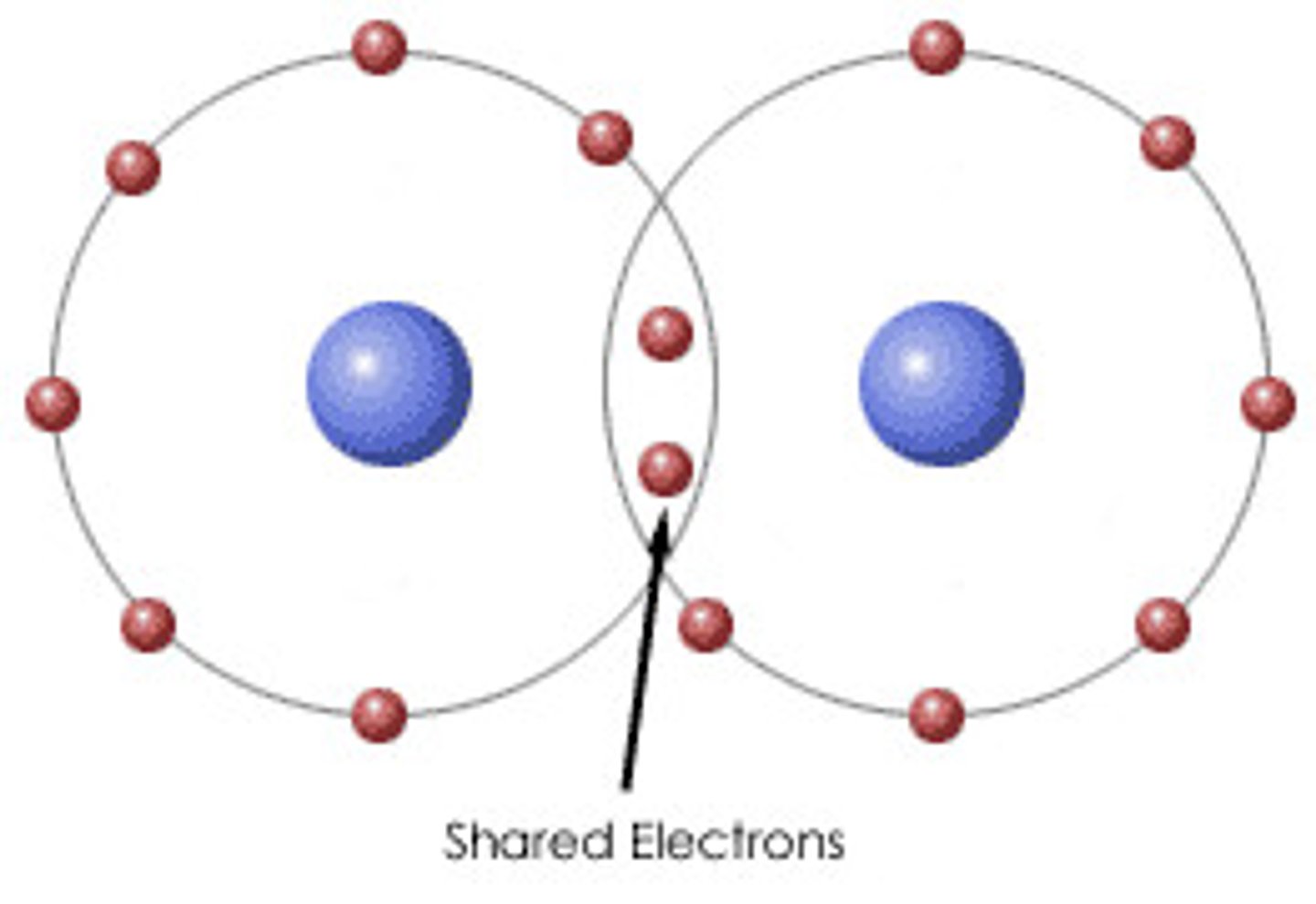
covalent bond
a type of strong bond between two or more of the same or different elements; forms when electrons are shared between elements
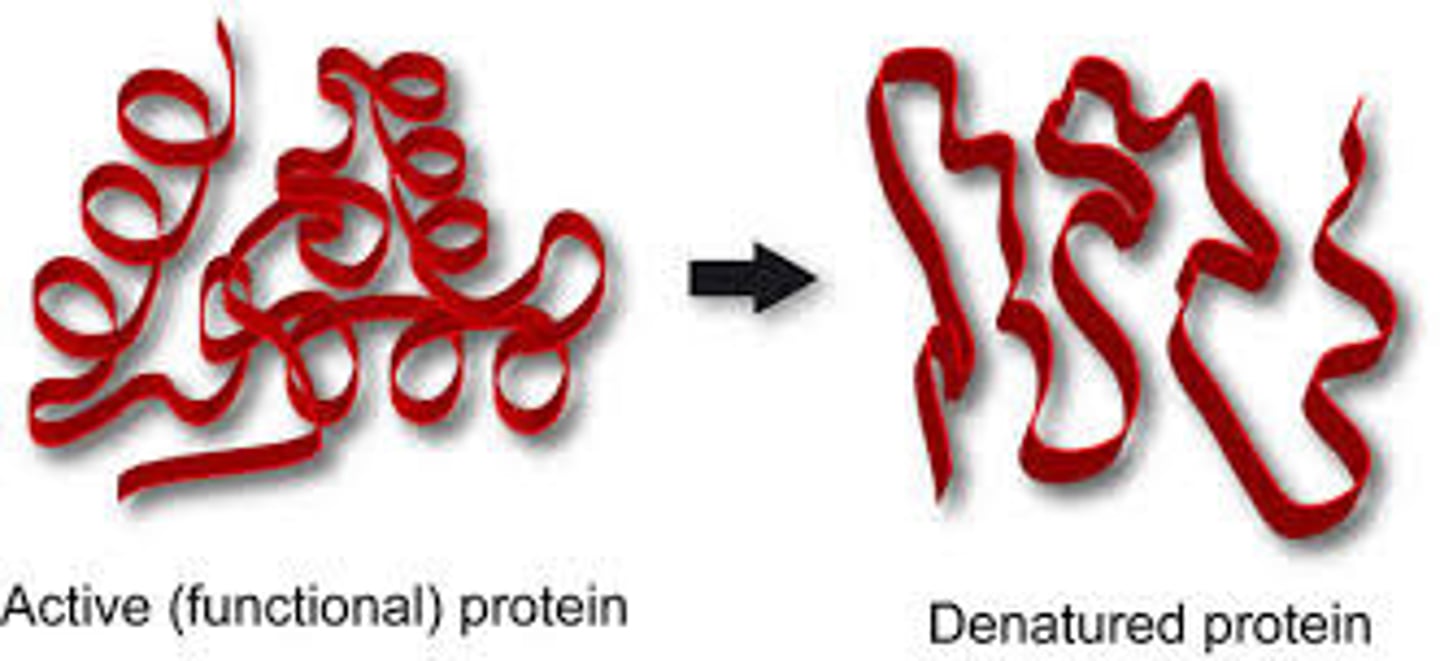
denaturation
the loss of shape in a protein as a result of changes in temperature, pH, or exposure to chemicals
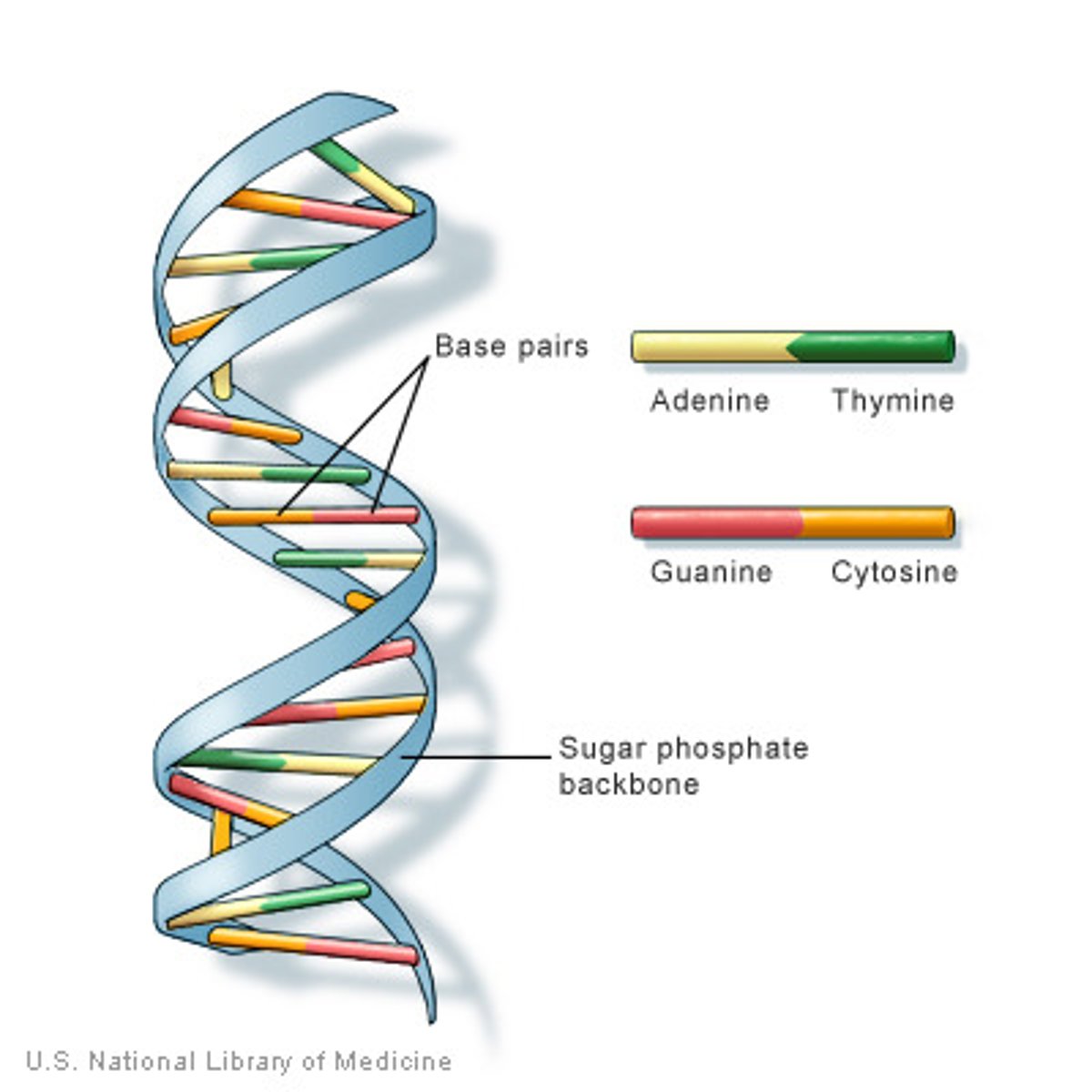
deoxyribonucleic acid (DNA)
a double-stranded polymer of nucleotides that carries the hereditary information of the cell
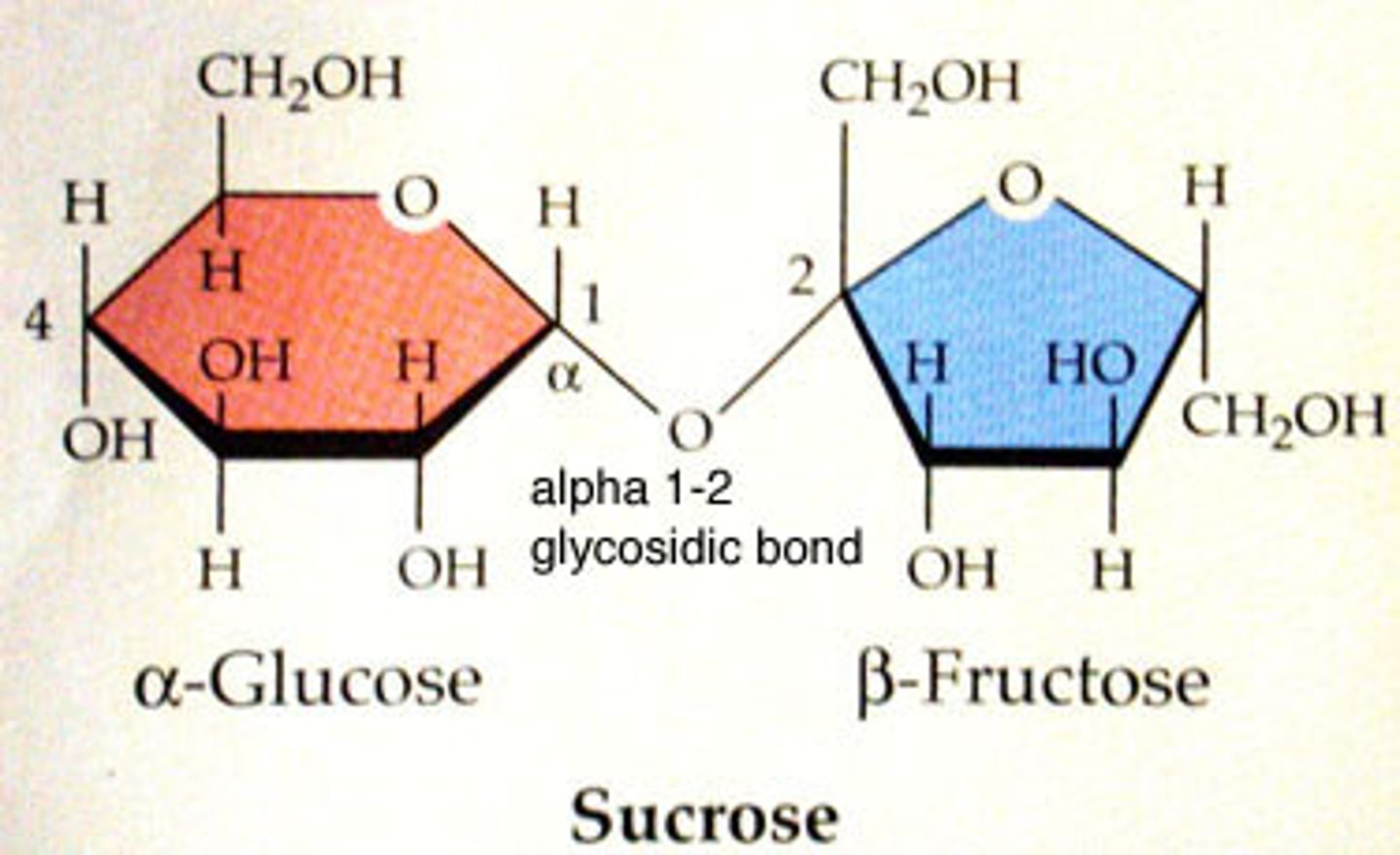
disaccharide
two sugar monomers that are linked together by a glycodsidic bond
electron
a negatively charged particle that resides outside of the nucleus in the electron orbital; lacks functional mass and has a charge of -1
electron transfer
the movement of electrons from one element to another
element
one of 118 unique substances that cannot be broken down into smaller substances and retain the characteristic of that substance; each element has a specified number of protons and unique properties
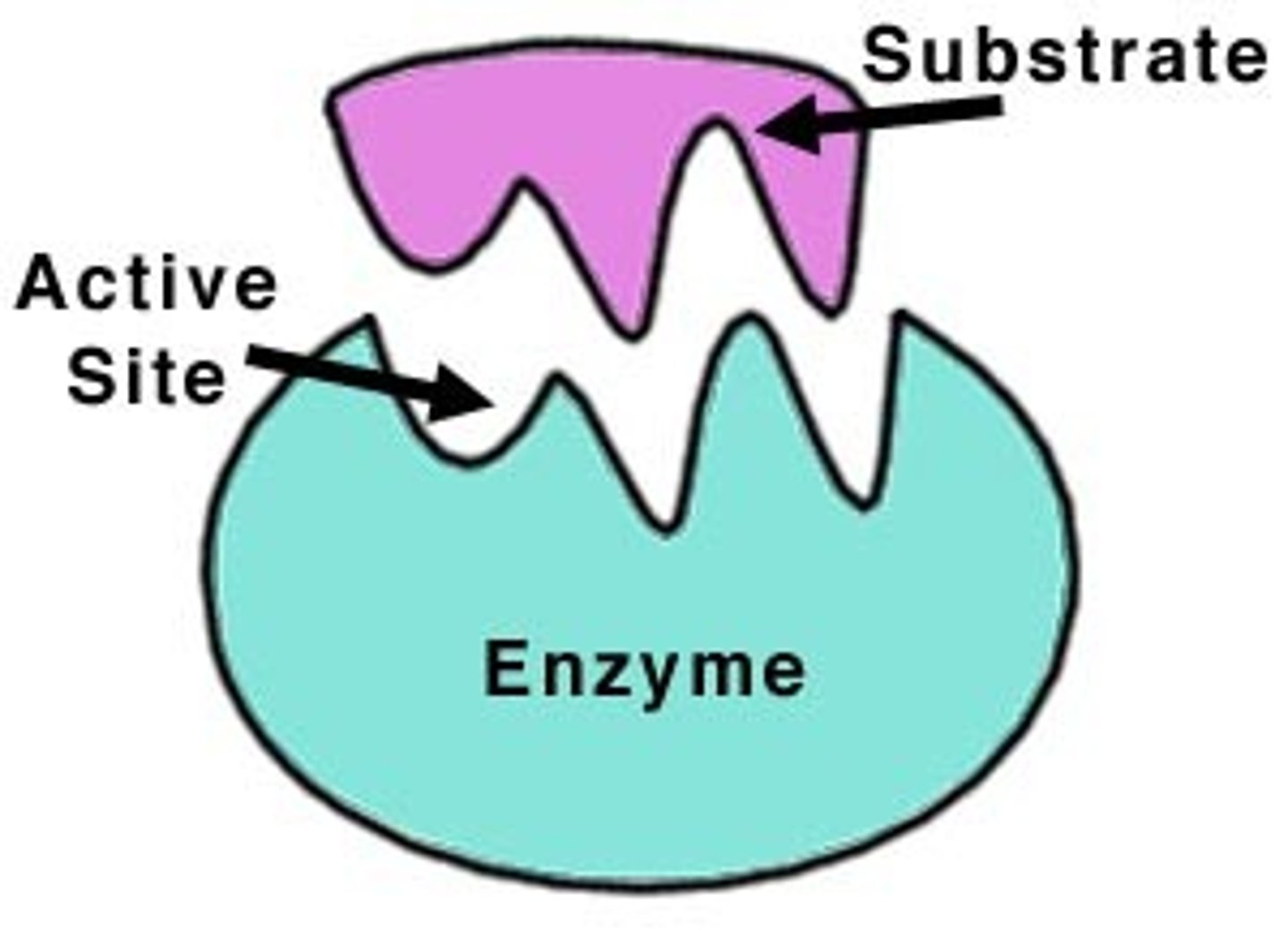
enzyme
a catalyst in a biochemical reaction that is usually a complex or conjugated protein
evaporation
the release of water molecules from liquid water to form water vapor

fat
a lipid molecule composed of three fatty acids and a glycerol (triglyceride) that typically exists in a solid form at room temperature

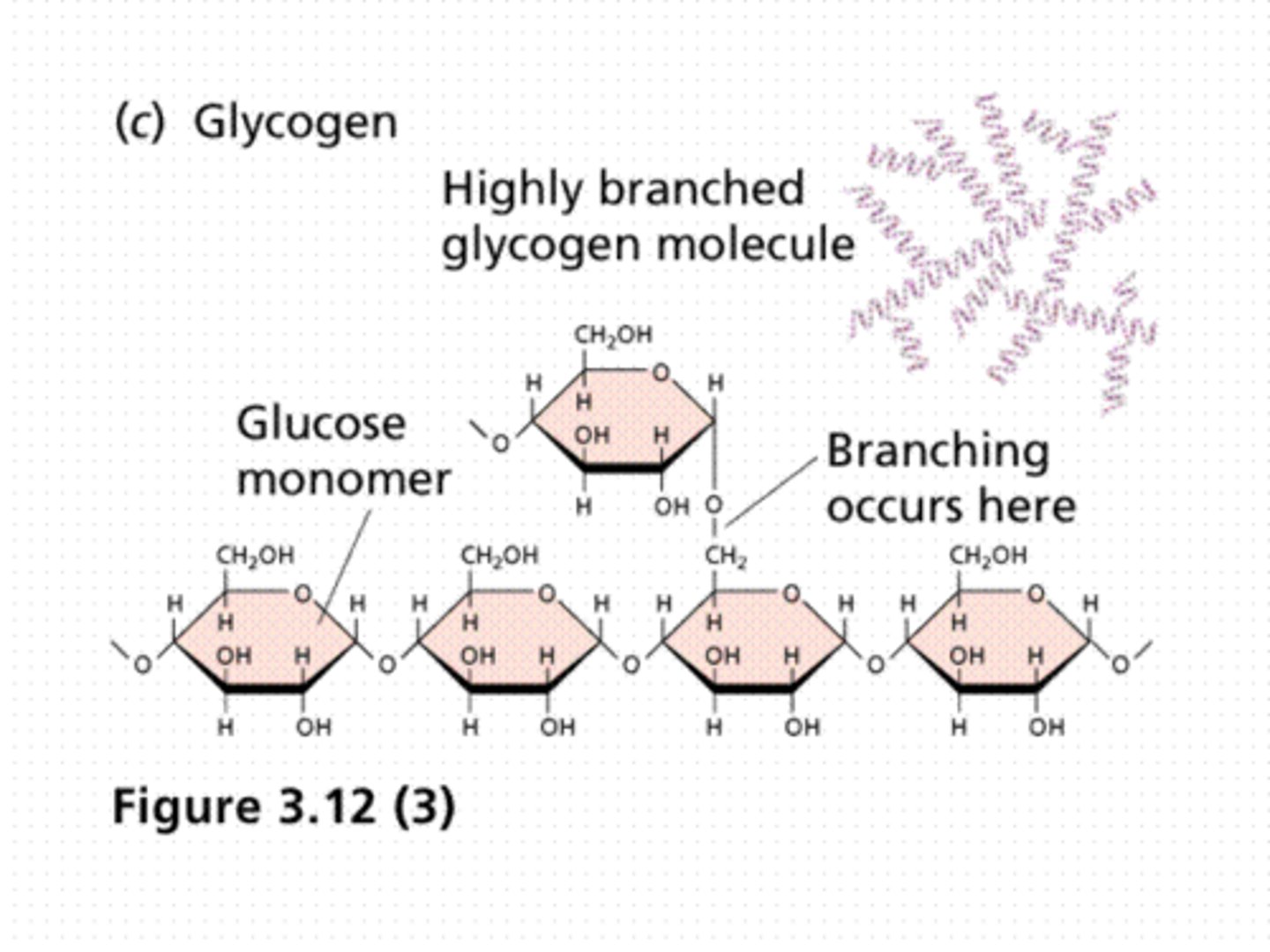
glycogen
a storage carbohydrate in animals
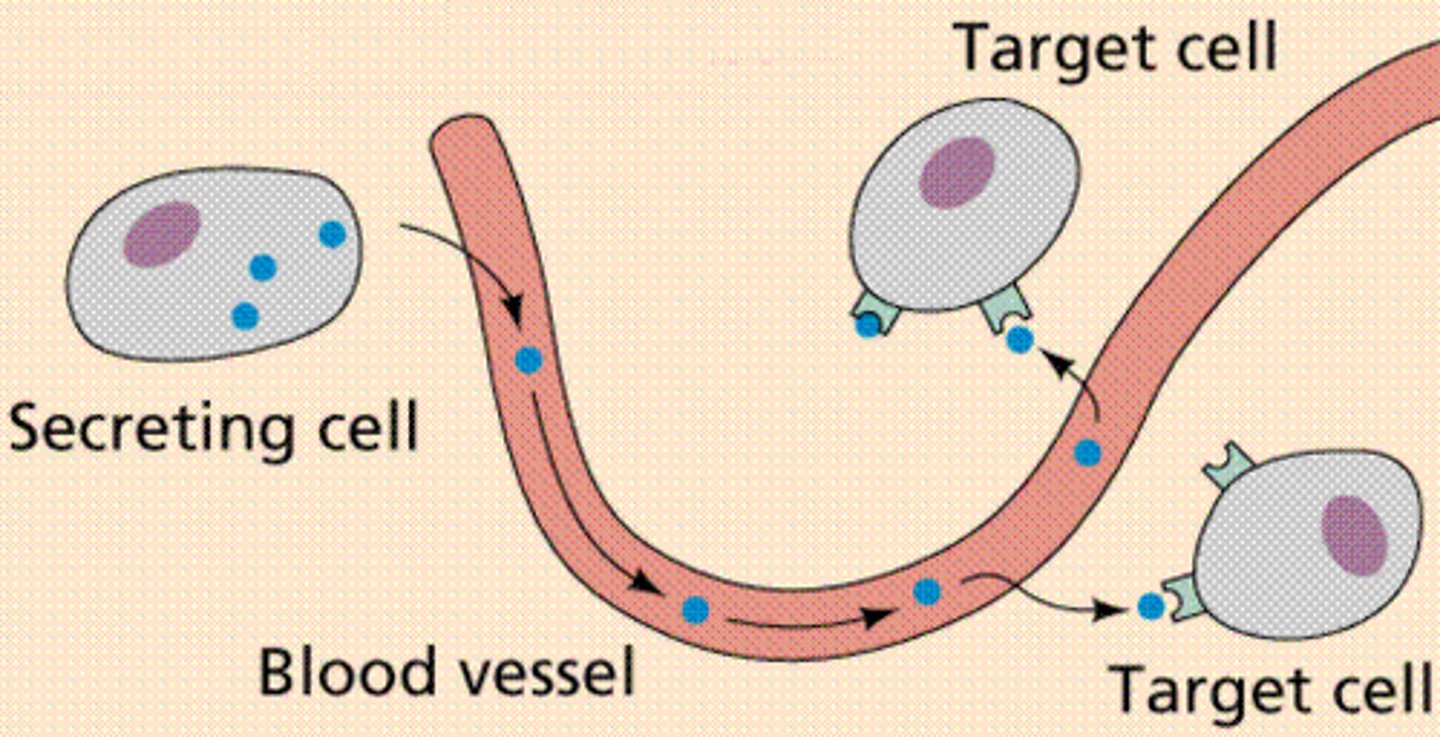
hormone
a chemical signaling molecule, usually a protein or steroid, secreted by an endocrine gland or group of endocrine cells; acts to control or regulate specific physiological processes
matter
occupies space and has matter, all mass is composed of elements
skip
skip
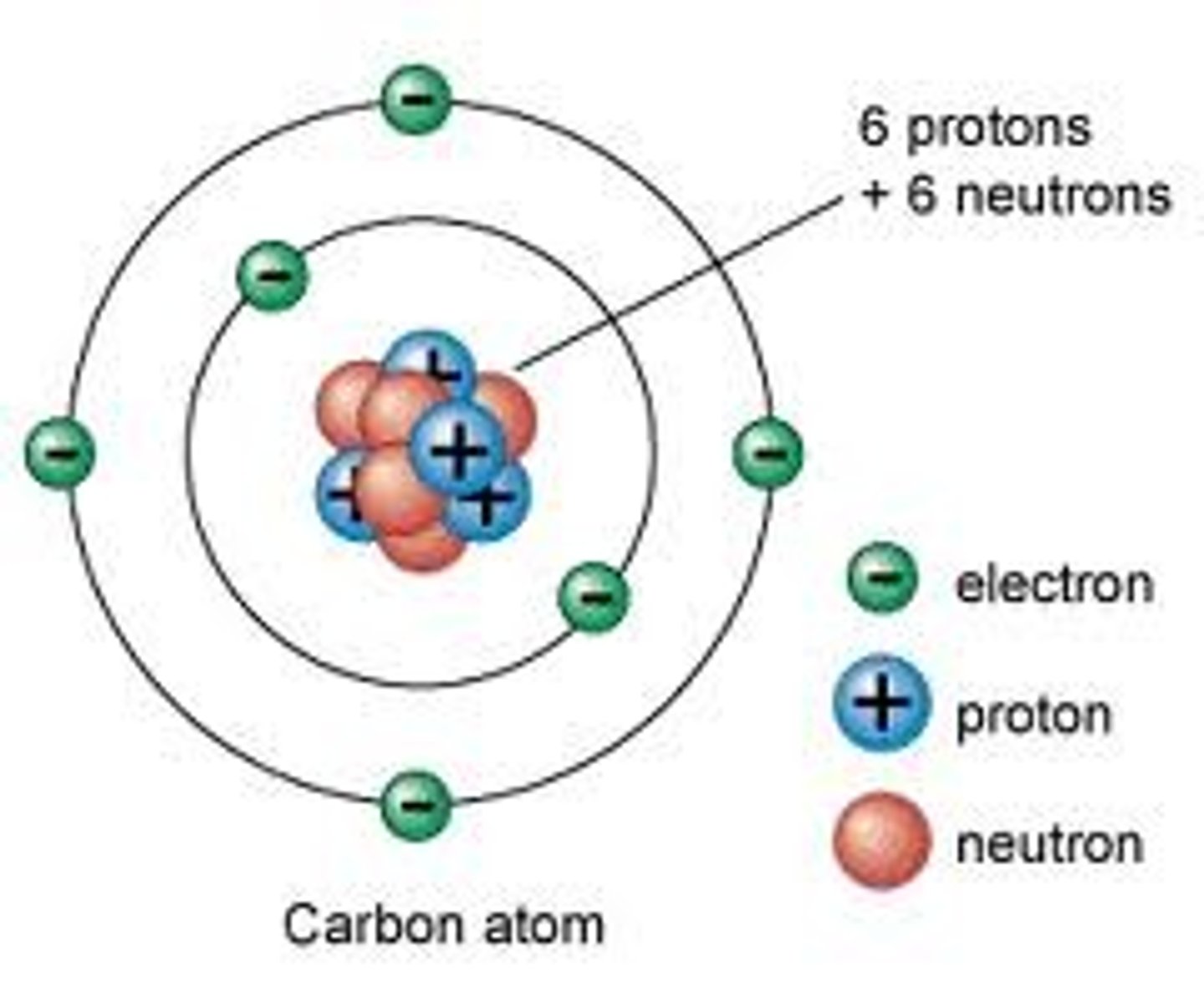
atom
the smallest component of an element that retains all of the chemical properties of an element
Ex. One hydrogen has all properties of the element hydrogen, such as it exists as a gas at room temperature, and it bonds with oxygen to create a water molecule
Hydrogen atoms cannot be broken down into anything smaller, while still having the
properties of hydrogen (like subatomic particles)
proton
positively charged particles that reside in the nucleus (core of an atom) of an atom and has mass of 1 and charge of +1.
Neutrons
like protons, reside in the nucleus of an atom. They have a mass of 1 and no charge. The protons and electrons charges balance its charge, (zero charge)
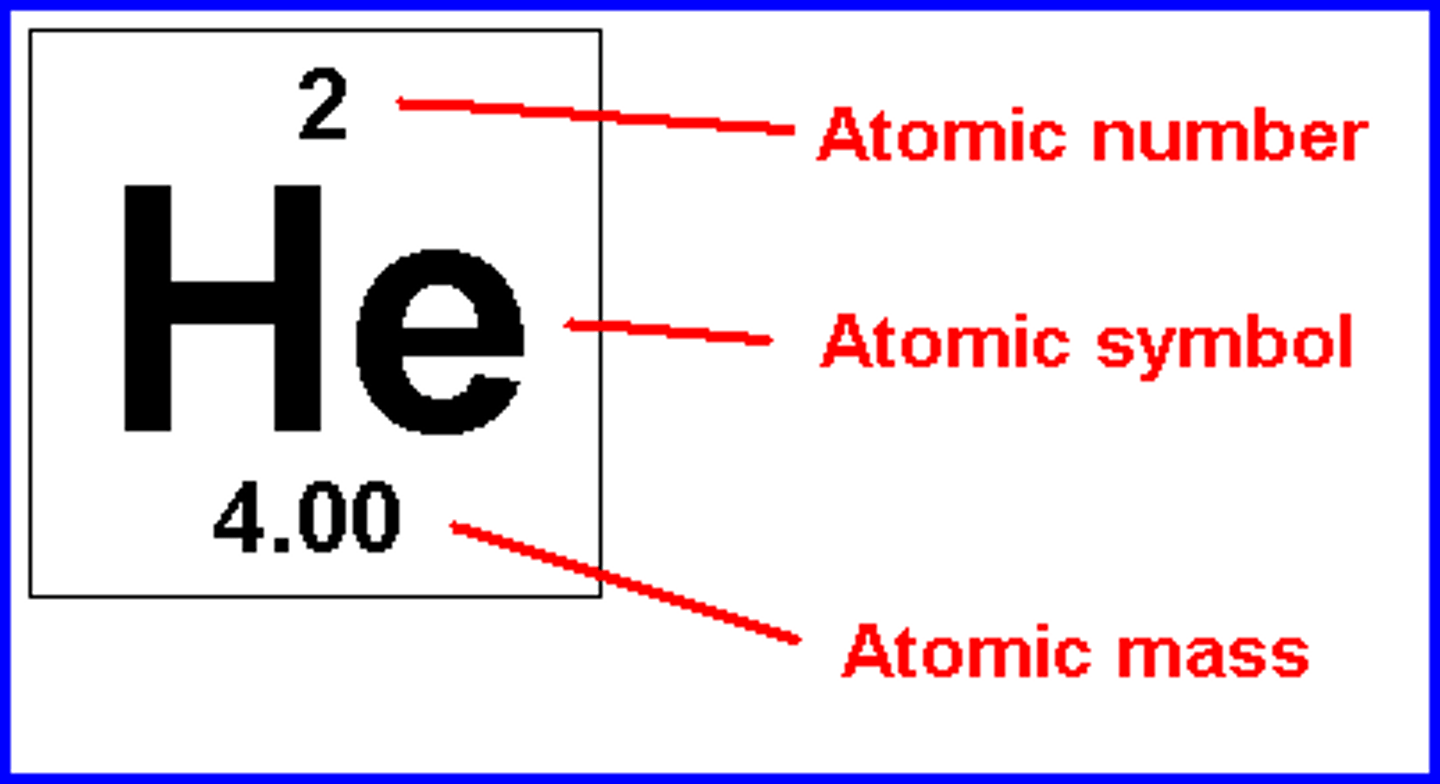
Atomic Mass
number of protons and neutrons of an element
Atomic number
number of protons in an element
Periodic table of elements
a chart of elements that include the atomic number and atomic mass
Provides key information about elements, often indicated by color-coding
Arrangement of the table shows how the electrons in each element are organized and provides how atoms will react with each other to form molecules.
Isotopes
different forms of the same element that have the same number of protons, but a different number of neutrons
Radioactive isotopes
are unstable, losing protons, subatomic particles, or energy to form more stable elements.
Chemical Bonds
Interactions between two or more of the same or different that result in the formation of molecules.
To gain greater stability, atoms will tend to completely fill their outer shells and will bond with other elements to accomplish this goal by sharing electrons, accepting electrons from another atom, or donating electrons to another atom
Octet Rule
an element can donate, accept, or share electrons with other elements to fill its outer shell
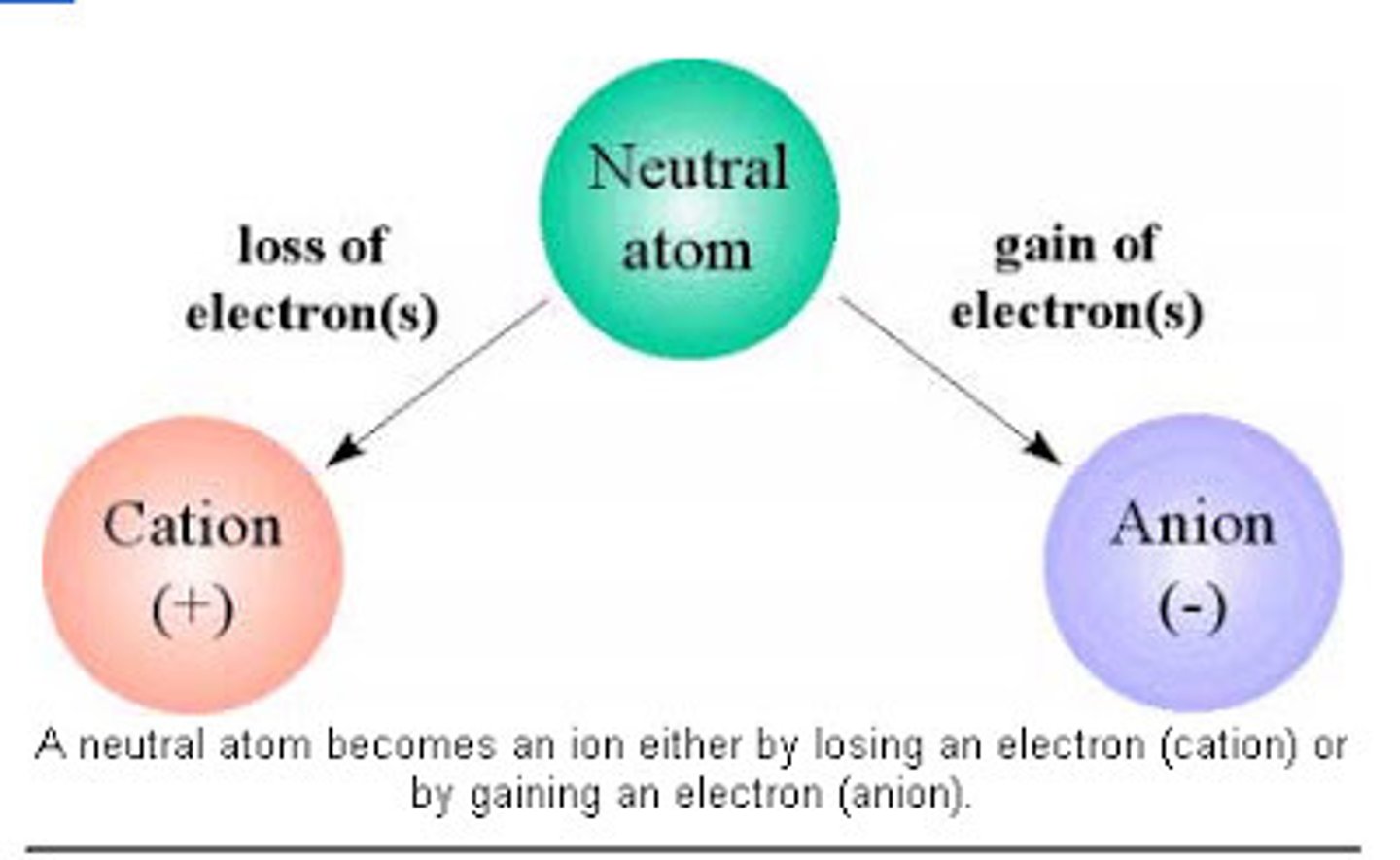
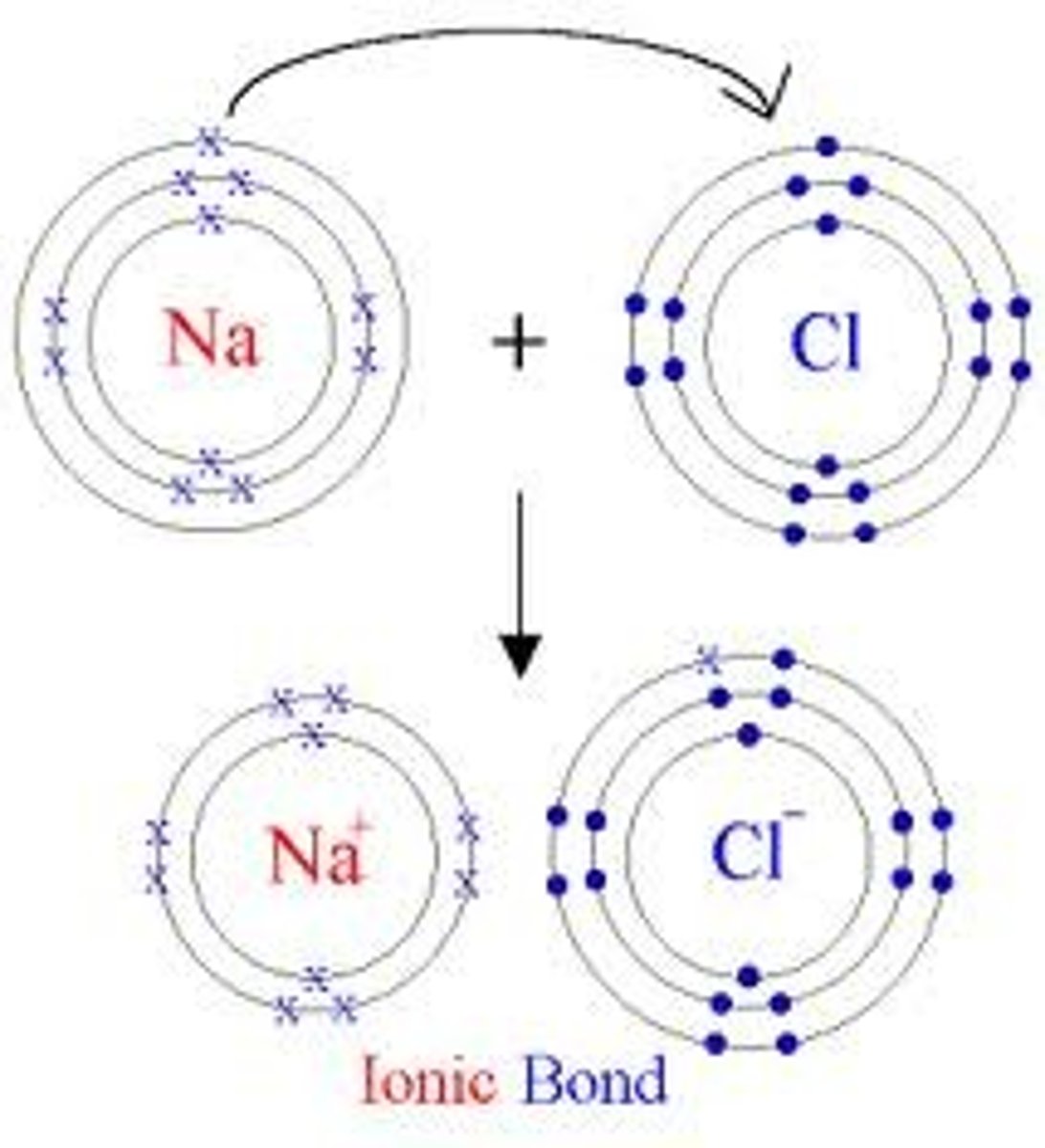
Ion
atoms that does not contain equal numbers of protons and electrons
Each has a net charge:
Positive ions- formed by losing electrons called cations
Negative ions- formed by gaining electrons, called anions
Electron Transfer
movement of electrons from one element to another
Ionic Bond
positive and negative charges attract, these ions stay together by bonding over the electron from one element staying predominantly with the other element.
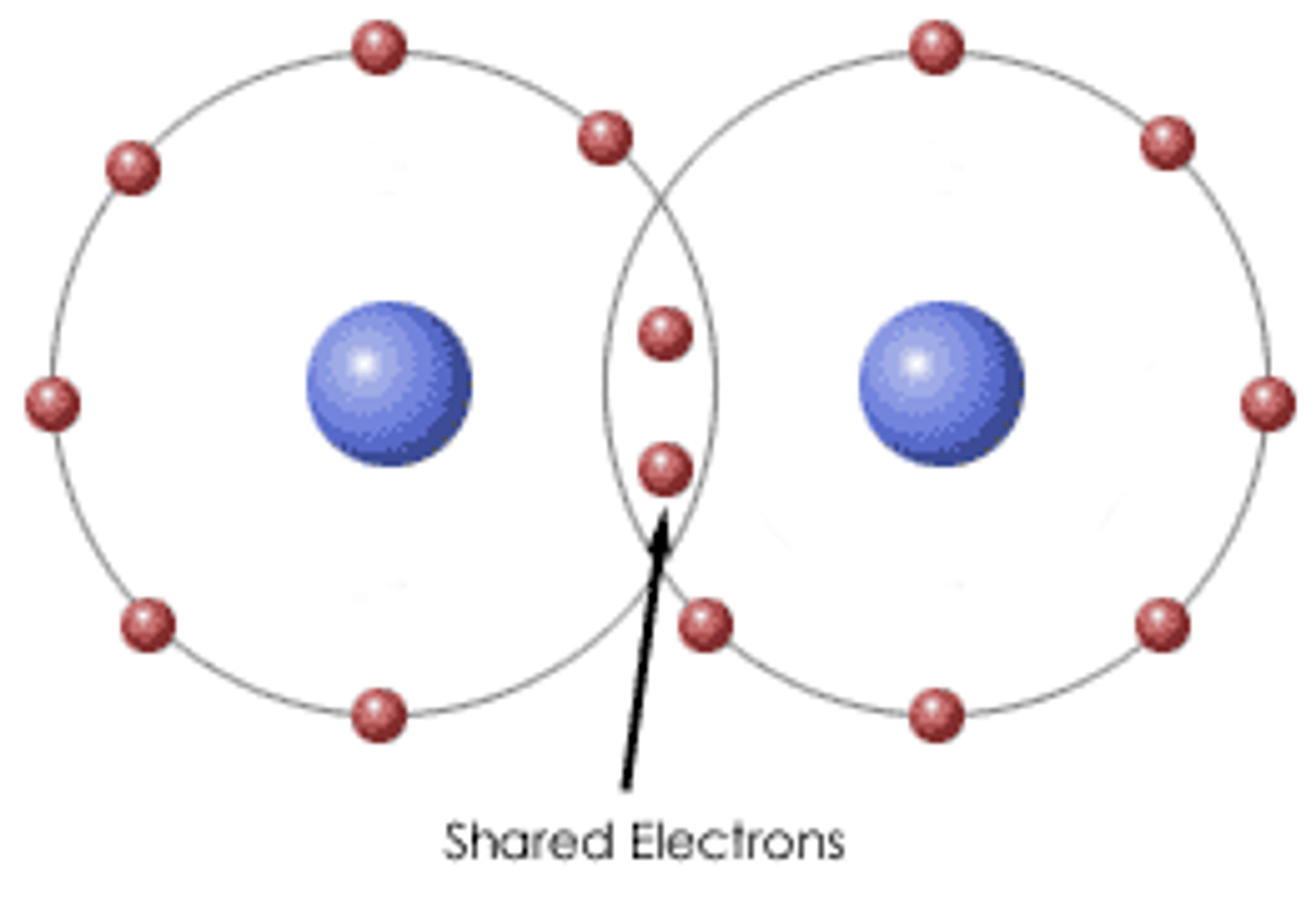
Covalent Bond
-formed when an electron is shared between two elements and is the strongest and most common form of chemical bond in living organisms.
-Forms between the elements that make up the biological molecules in our cells.
Covalent vs Ionic
Covalent bonds do not dissociate in water
Non-Polar
form between two atoms of the same element or between different elements that share the electrons equally.
Ex. Oxygen-Oxygen = covalent bond (2 electrons) to fill outer shell
Nitrogen-Nitrogen=Triple covalent bond (3 electrons)
Polar Covalent
electrons shared by atoms spend more time close to one nucleus than to the other nucleus
Unequal distribution between different nuclei causes a slightly positive or slightly negative charge.
Ex. Covalent bonds between oxygen and hydrogen atoms in water are polar covalent bonds. The shared electrons spend more time near oxygen nucleus, small negative charge, then they spend near hydrogen nuclei, giving these molecules a small positive charge
Hydrogen Bonds
two weak bonds that occur frequently
Attractions between positive and negative charges that do not require much energy to break
EX. Hydrogen Bond- when polar covalent bonds contain a hydrogen atom form, the hydrogen atom in the bond has a slightly positive charge.(The shared electrons are pulled more strongly to the binding of S+ charge of the hydrogen atom of one molecule and S- of another molecule. (without hydrogen bonding, water would be has and not liquid at room temperature)
Can form between different molecules and they do not always have to include a water molecule.
Hydrogen atoms in polar bonds within any molecule can form bonds with adjacent molecules
Hydrogen bonds can hold together 2 long strands of DNA, giving double standard structure. Hydrogen is also responsible for proteins' three-dimensional structure.
Van der Waals Interactions
weak attractions between molecules
They occur between polar, covalently bonded, atoms in different molecules.
Some of these weak attractions are caused by temporary partial changes formed when electrons move around a nucleus.
Some of these weak attractions are caused by temporary partial changes formed when electrons move around a nucleus (Important in biological systems
what bond does hydrogen and oxygen within water form?
polar covalent bonds
he shared electrons spend more time with oxygen atoms than hydrogen atoms. This creates S+ (H) and S- (O).
S+ (H) repel each other
Each water molecule attracts other water molecules because of the S+ and S- charges in different parts of a molecule.
Hydrophillic
“water loving”
When a substance readily forms hydrogen bonds with water, it can dissolve in water and it is referred to as hydrophilic. (water loving)
hydrophobic
When a substance is not readily formed with nonpolar substances like oils and fats
Why does water stabilize temperature?
The hydrogen bonds in water allow it to absorb and release heat energy more slowly than many other substances.
What is the measure of motion within molecules?
Temperature
As the motion increases, energy is higher, temperature is higher, this disrupts the hydrogen bonds between water molecules.
Because these bonds can be created and disrupted rapidly, water absorbs an increase in energy, temperature changes only minimally (water molecules temperature changes in the environment and within organisms)
Evaporation
as energy input continues, the balance between hydrogen-bond formation and destruction, swings more towards the destruction side.
More bonds are broken than formed, this results in the release of individual water molecules at the surface of the liquid
Ex. Evaporation of sweat, allows cooling of an organism
If the molecule decreases and temperatures drop, less energy is present to break the hydrogen bonds between water molecules.
When bonds remain intact and form a rigid, lattice-like structure, ice forms
Ex. Ice acts like insulation for animals and plant life in water
Solvent
a substance capable of dissolving another substance
The charged particles will form hydrogen bonds with a surrounding layer of water molecules, referred to as a sphere of hydration, keeping particles separated or dispersed in the water.
**water is an excellent solvent
Cohesion
water molecules are attracted to each other (due to hydrogen bonding), keeping molecules together at gas interface.
Surface Tension
the capacity of a substance to withstand rupture when placed under tension or stress
Adhesion
attraction between water molecules and other molecules
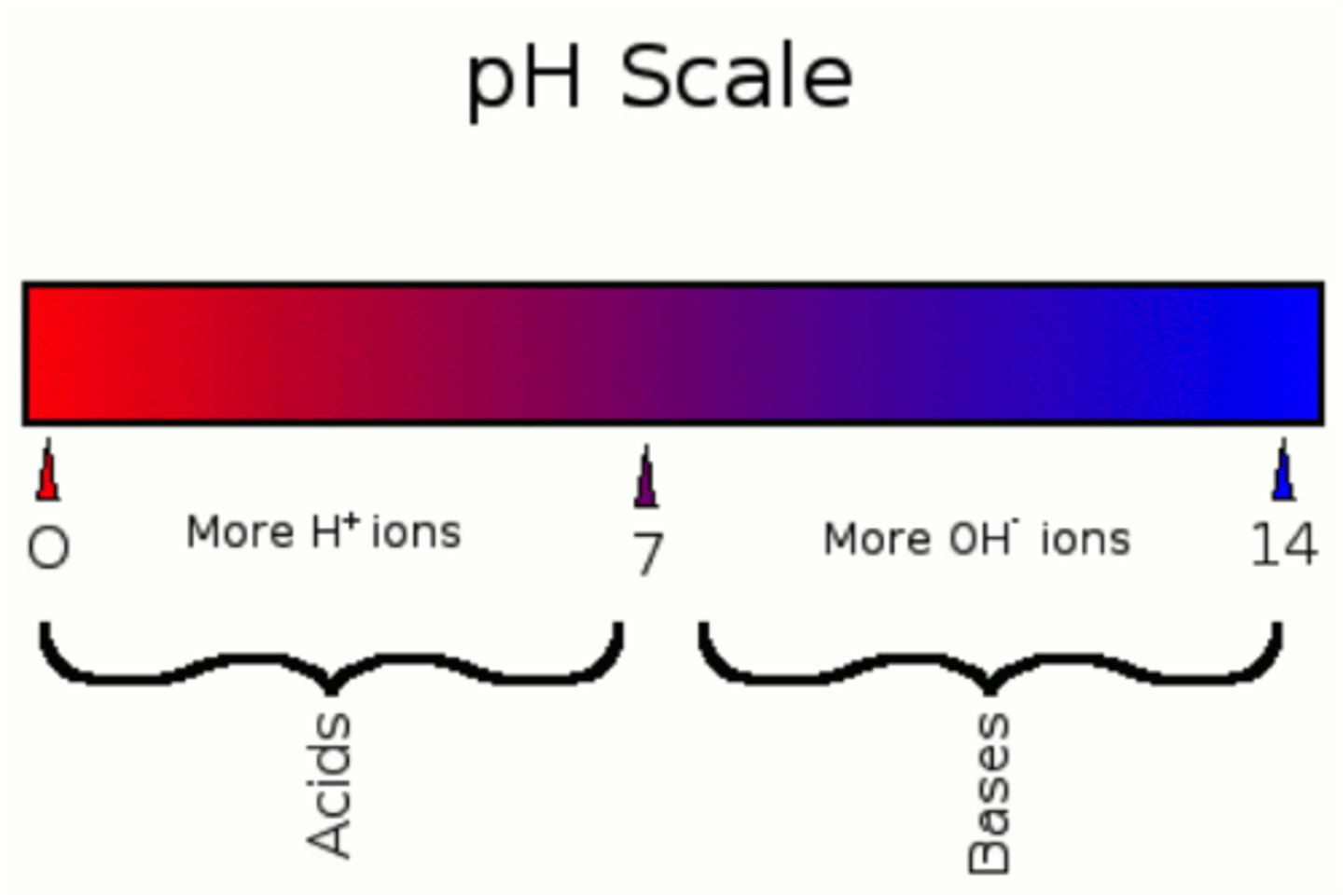
pH
the measure of acidity or basicity
pH test
measures the amount of hydrogen ions that exists in a given solution
High concentration ions yield low pH, low levels of hydrogen ions yield high pH
pH scale
0-14, a change in one unit is a factor of 10, 2 units = 100, etc
Pure water is neutral, pH of 7
Acids
substances that provide hydrogen ions and lower pH
Bases
provided hydroxide ions, raise pH
Buffers
absorb excess H+ or OH-, maintaining the body in the right pH range
Ex.Carbon Dioxide
What are the four types of biological compounds?
carbohydrates, nucleic acids, lipids, and proteins
All of these compounds are composed of different monomers, which can be combined to form unique polymers.
Ex. protein= amino acid (monomer),when a specific number of them are combined in a certain order, a unique protein is made.
Carbon backbone
the series of carbon atoms bonded together that forms the main structural chain of an organic molecule
Hydroxyl organic compound
a hydrogen bound to an oxygen on the carbon backbone
Carbonyl organic compound
a carbon linked by a double bond to an oxygen
Carboxyl organic compound
a carbon double-bonded to both an oxygen and hydroxyl group.
Amino organic compound
a nitrogen bonded to two hydrogen atoms.
Phosphate organic compound
phosphorus bonded to three oxygen atoms
Simple carbohydrates
compounds containing carbon, hydrogen, and oxygen in exact ratios (CnH2nOn), referred to monosaccharides
Monosaccharides can be combined to form other carbohydrates such as disaccharides, oligosaccharides, and polysaccharides.
what are the three types of monosaccharides?
glucose, fructose, galactose, all with formula C6H12O6, isomers of each other ( no one can be converted into another), They are primarily used as a source of energy production in cells.
Short chain carbohydrates
two classes: disaccharides and oligosaccharides
All disaccharides are composed of glucose, but each are unique due to the second monosaccharide added to it:
Lactose (glucose + galactose) present in milk
Sucrose (glucose + fructose) transport form of sugar used in plants and harvested by humans for food
Maltose (two glucose) present in germinating seeds
An oligosaccharide
a short chain of three or more glucose monomers
Ex. Raffinose and Stachyose, found in beans
Complex Carbohydrates
long chains of glucose, these chains may be branded or unbranded in their configuration
Polysaccharide- straight or branched chain of hundreds or thousands of sugar monomers.
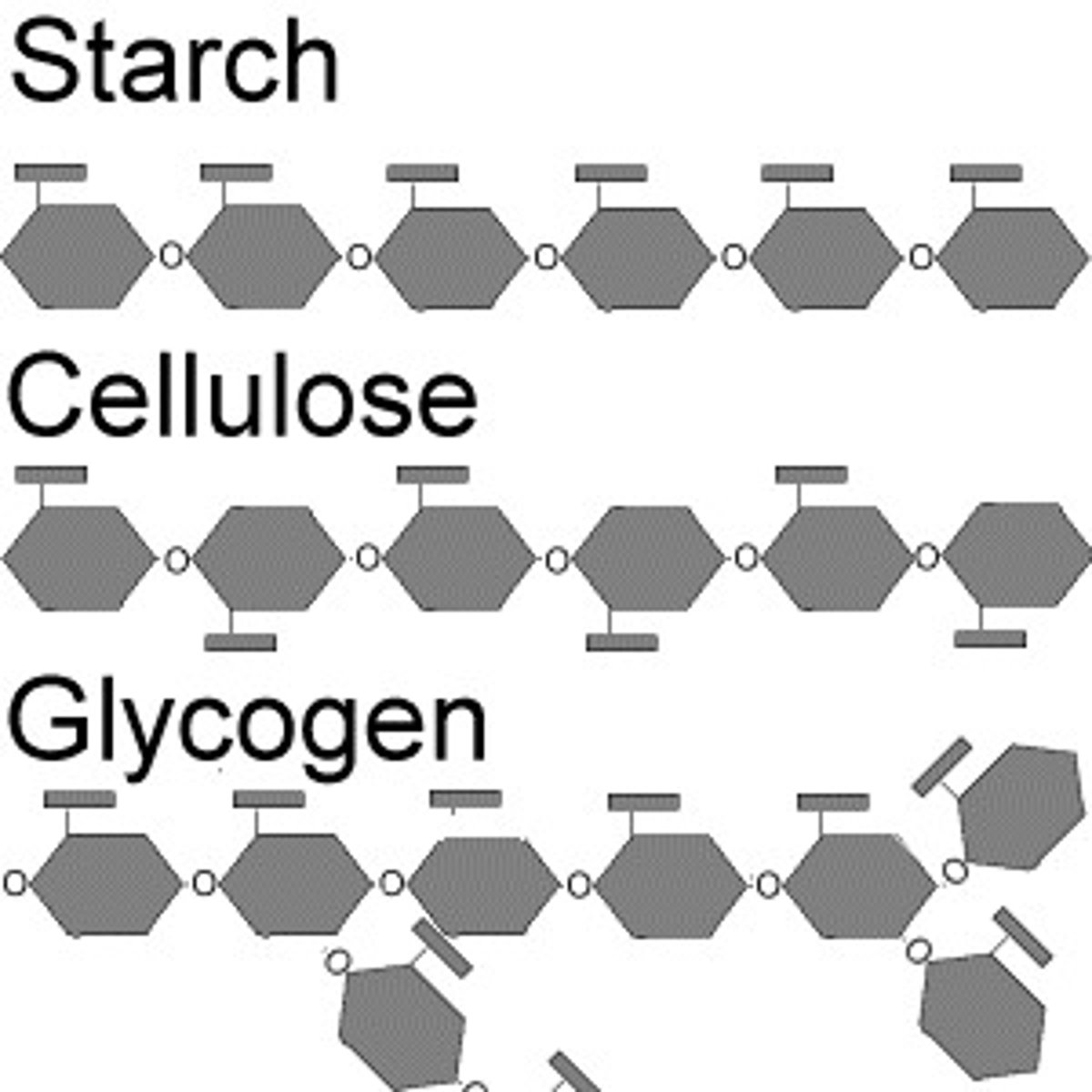
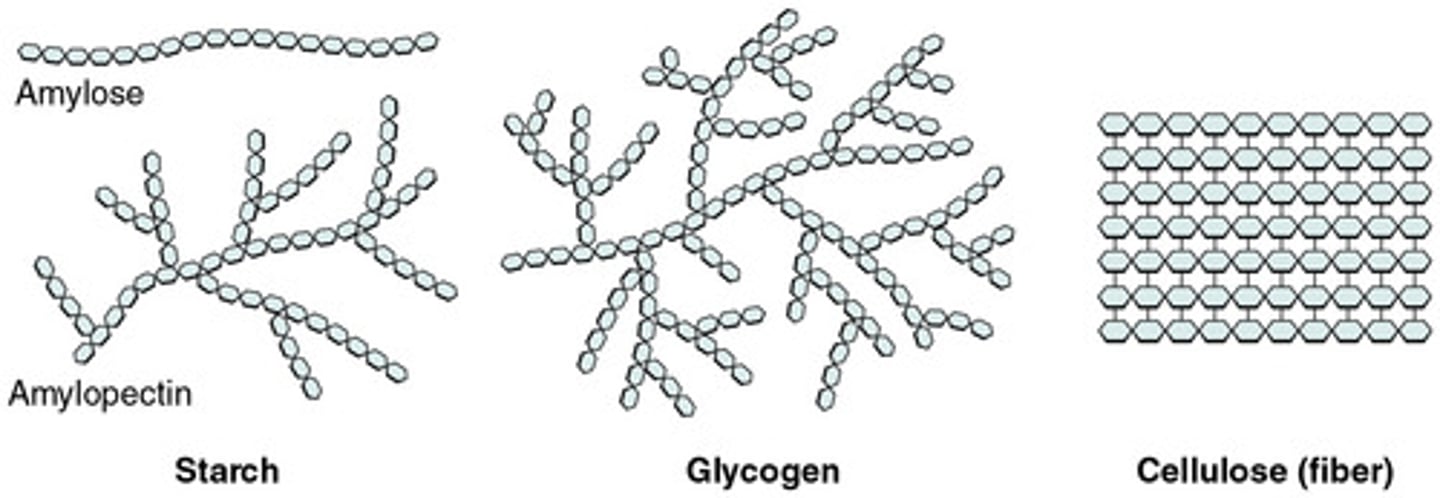
Starch- plant storage form of energym arranged as un-branched coiled chains, easily hydrolyzed to glucose units.
Cellulose- source of dietary fiber-tough, insoluble-used in plant cell walls.
Pectin- source of dietary fiber, insoluble, used in plant cell walls
Glycogen- a highly branched chain, used by animals to store energy in muscles and liver.
Chitin- specialized polysaccharide with nitrogen attached to glucose units, used as structural material in arthropod exoskeletons and fungal cell walls.
Lipids
greasy/oily compounds with little tendency to dissolve in water (non-polar/hydrophobic)
They can be broken down by hydrolysis reactions and created by condensation reactions.
Functions in energy storage, membrane structure, and coatings.
Triglycerides
a fatty acid, a long chain of mostly carbon and hydrogen atoms with a carboxyl group at one end.
Saturated (triglycerides) fatty acids
have only single c-c bonds in their fatty acid tails
Fats are formed by the attachment of one (mono), two (di-) or three (tri-) fatty acids to a glycerol.
Unsaturation fatty acids
liquids (oils) at room temperature because one or more double bonds between the carbons in the fatty acids permit “kinks” in the tails.
It can also have a cis or trans configuration: the double bond in a cis fatty acid can cause the fatty acid to bend, whereas the bond in a trans fatty acid enables the fatty acid to maintain a linear (non-bent) configuration.
what has a rich source of energy, yielding more than twice the per weight basis as carbohydrates + provides an insulation blanket for animals that must endure cold, harsh temperatures.
Saturated and Unsaturated fatty acids
Phospholipids
composed of two fatty acids and a phosphate group; both bound a carbon backbone referred to as a glycerol. They are the main structural material of cell membranes, arranged in bilayers.
Sterols and their Derivatives
sterols have a backbone of four carbon rings but no fatty acid tails are considered a subgroup of steroids.
Ex. Cholesterol is a sterol and found in plants and animals.
Waxes
formed by attachment of long-chain fatty acids to long-chain alcohols or carbon rings and serve as coatings for plant parts and as animal coverings.
Proteins
most diverse of all biological molecules
What are the functions of proteins?
catalysts (enzymes), cell movement, storage and transport agents, hormones, antibodies, structural material
What is the monomer unit of proteins?
amino acid, contains central carbon that binds a hydrogen, an amino group, a carboxyl group, and one of twenty varying R groups.
Polypeptides
polymers of amino acids, reflect special peptide bonds that form between the amino acids.
What are the four levels of organization?
Primary structure- ordered sequences of amino acids each linked together by peptide bonds to form polypeptide chains.
Secondary Structure- helical coil (hemoglobin) or sheet-like array (as in silk) that results from hydrogen bonding of side groups on the amino acid chains.
Tertiary Structure- result of folding due to interactions among R groups along the polypeptide chain, sometimes called “supercoiling”
Quaternary Structure- describes the twisting of two or more polypeptide chains.
Denaturation
process of changing protein three-dimensional structure due to changes in protein’s biochemical and thermal environment.
Nucleic Acids
DNA (Deoxyribonucleic acid) and RNA (Ribonucleic Acid) are polymers of nucleotides, which are composed of three units:
5- carbon sugar (ribose or deoxyribose)
A nitrogen-containing base
A phosphate group
What are the four bases in DNA
Adenine
Cytosine
Guanine
Thymine (RNA uses Uracil)
What gives DNA’s helical shape?
two polynucleotide chains that are hydrogen bonded together
Hydrogen bonding occurs as a result of specific complementary pairing of nucleotide bases.