Module 11 - Biosynthesis of Cell Components
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
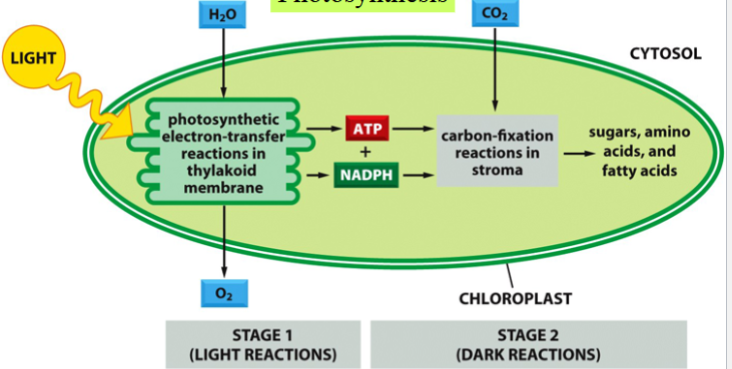
the two sets of reactions of photosynthesis
light reactions and dark reactions
both occur in chloroplast
photosynthesis
the conversion of sunlight energy into chemical energy by living organisms
end product: generation of organic molecules from atmospheric CO2
two stages: light and dark reactions
occurs in chloroplasts
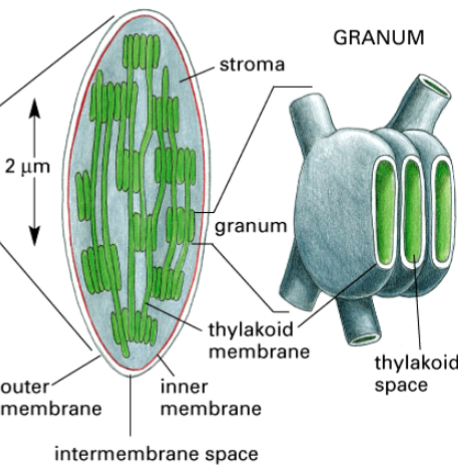
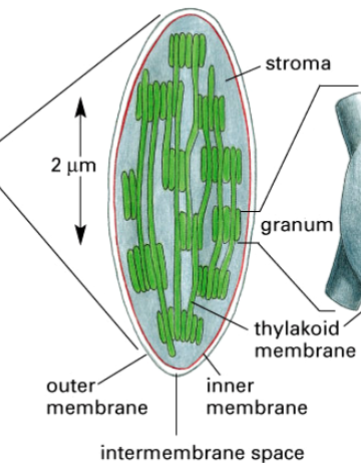
chloroplasts
organelles that conduct photosynthesis
found in plants and eukaryotic algae
consist of thylakoids (with thylakoid space), chlorophyll, stroma
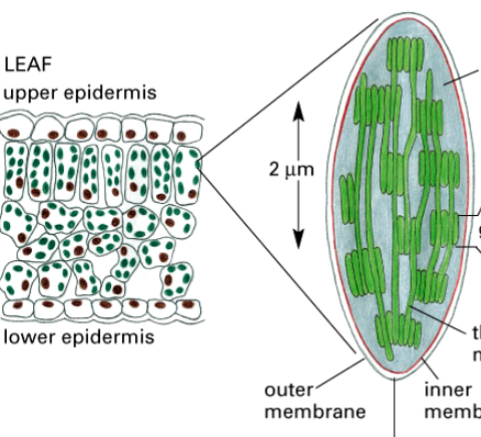
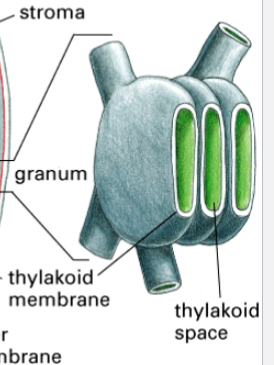
thylakoids
one component of a chloroplast
two membranes plus a third membrane system
has thylakoid space inside
within the chloroplast, they stack like coins
contain chlorophyll pigment
contains an electron transport, where a H+ gradient forms across it, to synthesize ATP
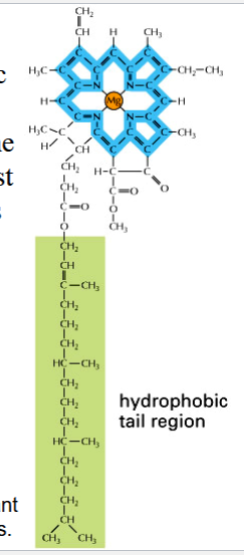
chlorophyll
one component of a chloroplast
green photosynthetic pigment in plants
found in photosystems of thylakoid membranes
yields a green color because it reflects green (absorbs other visible light wavelengths)
energy is absorbed from sunlight by electrons in a decentralized electron cloud → light energy is converted into chemical energy
chemical energy then jumps from chlorophyll to chlorophyll within the system
stroma
one component of a chloroplast
the space inside the chloroplast
thylakoid space
one component of a chloroplast
the space inside thylakoids
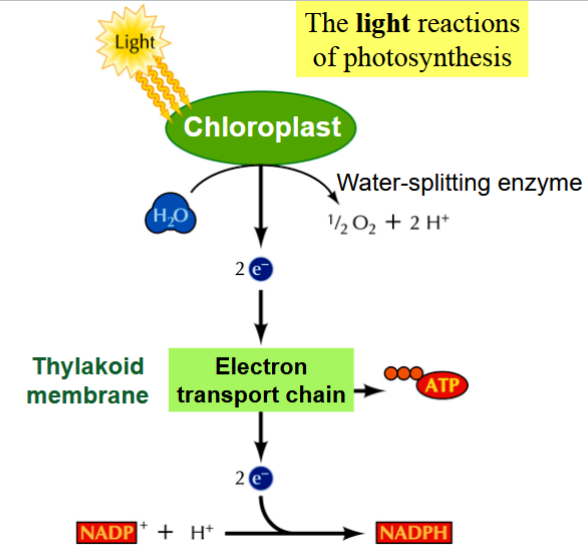
the light reactions of photosynthesis
H2O is split → ½O2 and 2H+ by a water-splitting enzymes
electrons are released from the splitting of H2O → get energized by sunlight
energized electrons move through an electron transport in the thylakoid membrane → a H+ gradient forms across the thylkaoid membrane
energy in the H+ gradient is used to synthesize ATP (similar to oxidative phosphorylation in mitochondria)
final electron is NADP+ → gets reduced to NADPH
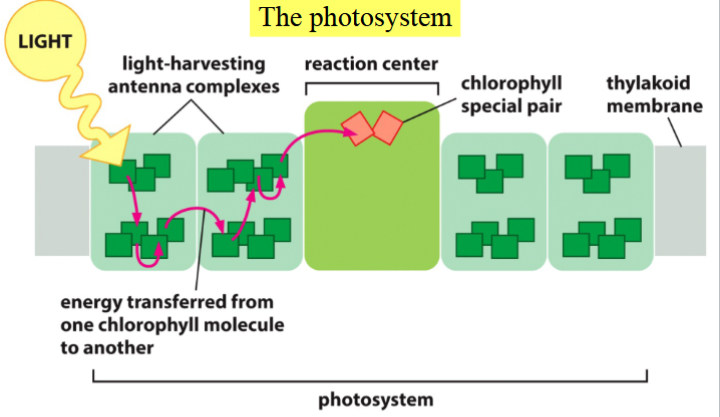
photosystem
a system within thylakoid membranes; part of the light reactions
has a central photosynthetic reactions center with a special pair of central chlorophylls that accept and donate electrons, and 100s of antenna chlorophylls that harvest sunlight energy
sunlight excites electrons in antenna chlorophylls into an unstable high-energy state
energy jumps from chlorophyll → chlorophyll until it reaches the two central chlorophylls
the central chlorophylls trap and donate the energy as energized electrons to an electron transport chain
difference between photosystems I and II (accepting electrons)
PI accepts electrons from plastocyanin
PII accepts electrons from water
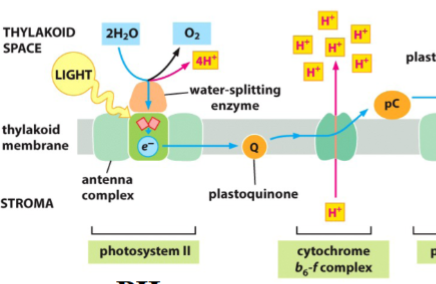
electron transport in photosystem II
energy is transferred through 100s of antenna chlorophylls to central chlorophylls and donated to an ETC
ETC: plastoquinone (Q), then cytochrome bf complex
a water-splitting enzyme extracts electrons from H2O to replace electrons donated by PSII (generates O2 and H+)
cytochrome bf complex: an H+ pump that uses energy of electrons to pump H+ into thylakoid space
electrons are carried to photosystem I by plastocyanin
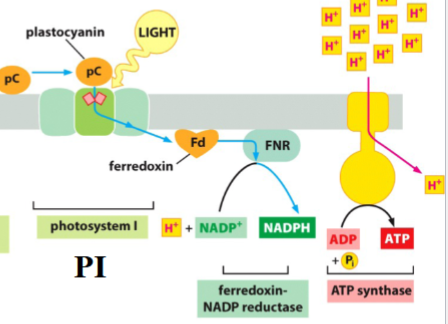
electron transport in photosystem I
electrons (from photosystem II) receive energy absorbed by 100s of antenna chlorophylls from sunlight → electrons are in the special pair of chlorophylls in the reaction center
energized electrons are donated to ferredoxin (Fd)
electrons donated are replaced with electrons from plastocyanin (pC)
ferredoxin-NADP reductase (FNR): synthesizes NADPH using electrons transferred from Fd to reduce NADP+ (the final electron acceptor)
ATP synthase uses H+ gradient to power ATP synthesis
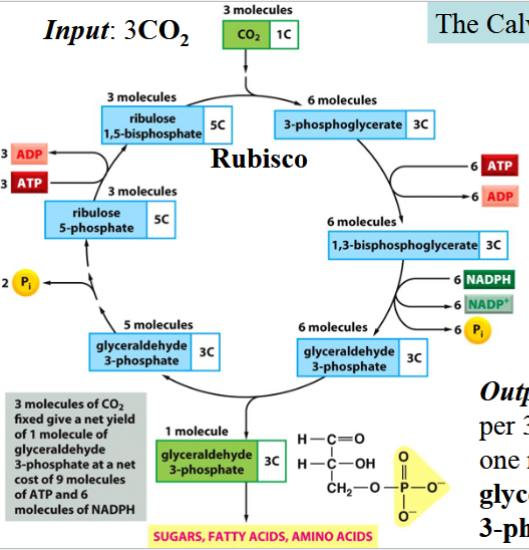
dark reactions of photosynthesis
aka the Calvin cycle
ATP and NADPH generated during light reactions are used to synthesize glucose
occurs in the stroma
carbon fixation: a CO2 is added to each of three molecules of ribulose 1,5-bisphosphate (RuBP)
yields 3 molecules of a 6-carbon unstable intermediate
^catalyzed by rubisco
intermediates split into 2 3-carbon molecules (each, so x2)
product: 6 molecules 3-phosphoglycerate, and incorporation of carbon into the living world
two cycles run: output is 2 molecules of glyceraldehyde 3-phosphate → synthesize 1 glucose
balanced equation of photosynthesis in plants
6CO2 + 6H2O → C6H12O6 + 6O2
gluconeogenesis
the synthesis of glucose from pyruvate
anabolic
most reactions are the reverse of glycolysis, but some of the enzymes needed are different for the irreversible steps (steps 1, 3, and 10 of glycolysis → steps 1, 8, and 10 in gluconeogenesis)
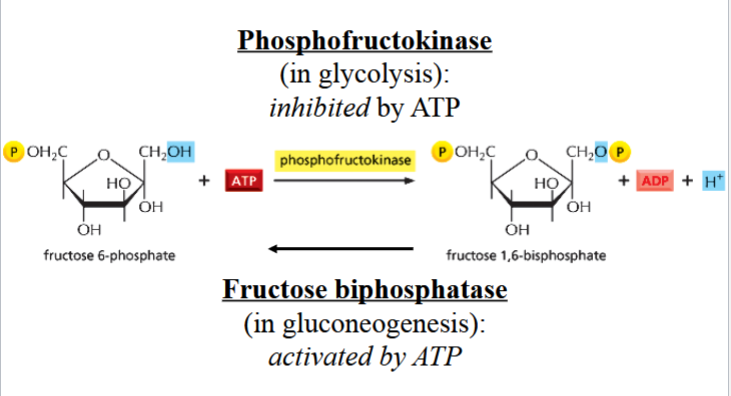
e.g. reverse reaction of glycolysis step 3 is catalyzed by fructose bisphosphatase, instead of phosphofructokinase
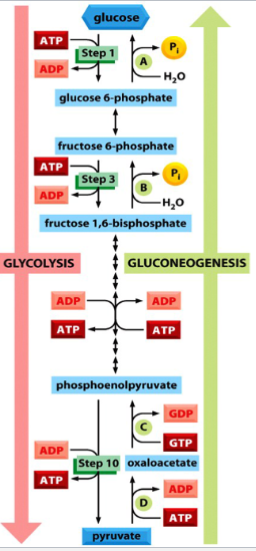
importance of different enzyme requirements in gluconeogenesis vs glycolysis
differences in enzyme requirements allows the cell to regulate glycolysis and gluconeogenesis independently
e.g. if ATP levels are high, glycolysis is decreased and gluconeogenesis is increased
e.g. if ATP levels are low, glycolysis is increased and gluconeogenesis is decreased
(ADP and ATP can be allosteric inhibitors or allosteric activators)
synthesis of nucleoside monophosphates
synthesis of subunits of nucleic acids
can be de novo or salvage
de novo nucleoside monophosphate synthesis
“from scratch”
long pathways that assemble nucleic acid subunits from smaller components
salvage nucleoside monophosphate synthesis
“recycling” of nitrogenous bases
short pathways to assemble nucleic acid subunits
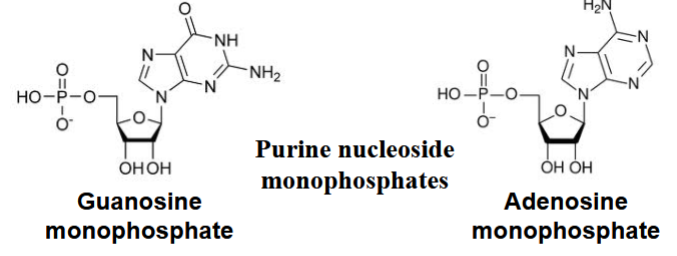
purine nucleoside monophosphate synthesis via salvage pathways (summarized from extra information)
a pyrophosphate is converted into many intermediates, to inosate
or hypoxanthine → inosate (with HGPRT as enzyme)
inosate: an intermediate nucleotide
if either guanine or adenine added to inosate, either G monophosphate or A monophosphate will be made
HGPRT deficiency
a problem in purine salvage pathways
if deficient, hypoxanthine converts to uric acid instead of inosine
uric acid precipitates as crystals in joints → triggers gout
if severe, results in Lesch-Nyhan syndrome
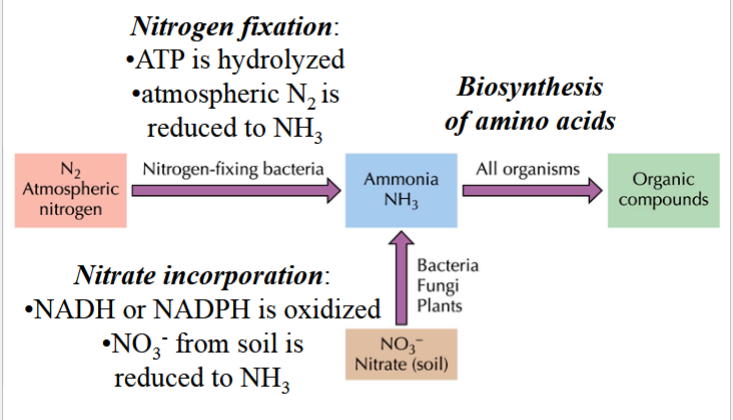
assimilation of nitrogen
N2 (atmospheric) gets reduced to NH3 (ammonia) via N-fixing bacteria, and ATP is hydrolyzed
NO3- (nitrate in soil) is incorporated (reduced) to NH3 by bacteria, fungi, and plants (NADH or NADPH is oxidized)
NH3 is used in biosynthesis of amino acids to make organic compounds in all organisms
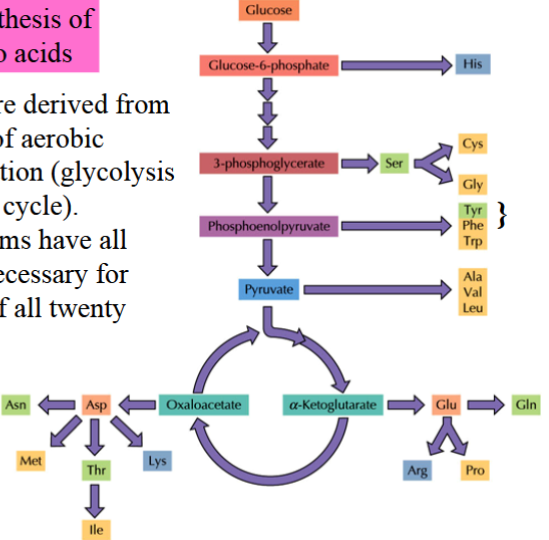
biosynthesis of amino acids
amino acids are derived from intermediates of aerobic cellular respiration (glycolysis and CAC)
there are twenty main amino acids
not all organisms have the enzymes necessary for synthesis of all twenty AAs
essential AAs must be obtained through diet
nonessential AAs can be synthesized (by humans)
the essential amino acids
histidine
isoleucine
leucine
lysine
methionine
phenylalanine
threonine
tryptophan
valine
must be obtained from dietary sources
the nonessential amino acids
alanine
arginine
asparagine
aspartate
cysteine
glutamate (glutamic acid)
glutamine
glycine
proline
serine
tyrosine
in humans, can be synthesized in the body
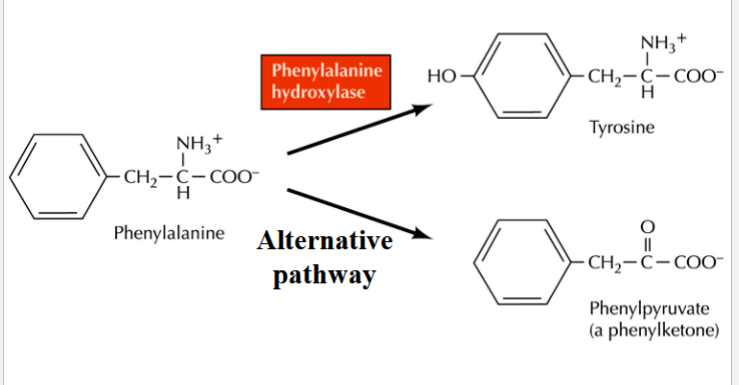
phenylketonuria
a deficiency of phenylalanine hydroxylase (enzyme that converts phenylalanine → tyrosine)
phenylalanine accumulates, gets deaminated to phenylpyruvate
children develop intellectual disability within the first year of life
can be prevented with newborn screening for elevated phenylalanine levels, and low phenylalanine diet (for mother while pregnant, and for child)
macromolecule synthesis
arrangement of free subunits into larger chains or complexes or macromolecules
nucleotides → nucleic acids
simple sugars → polysaccharides
amino acids → polypeptides
fatty acids and simple lipids → fats, lipids
require bond formation between subunits by dehydration synthesis
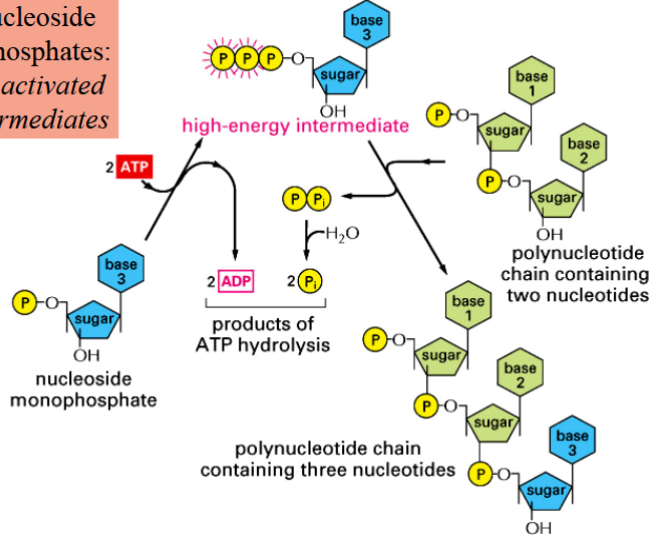
processes require synthesis of an activated (high-energy) intermediate before formation of the inter-subunit bond
nucleoside triphosphates are example of activated intermediates (energy from hydrolysis of these intermediates allows bond formation)
glucose storage
polysaccharides store glucose in larger units
glycogen in animals, starches and cellulose in plants
synthesis of polysaccharides involves glycosidic bond formation between simple sugars (requires energy)
synthesis is coupled with formation of a nucleotide sugar intermediate (activated, energy-yielding)
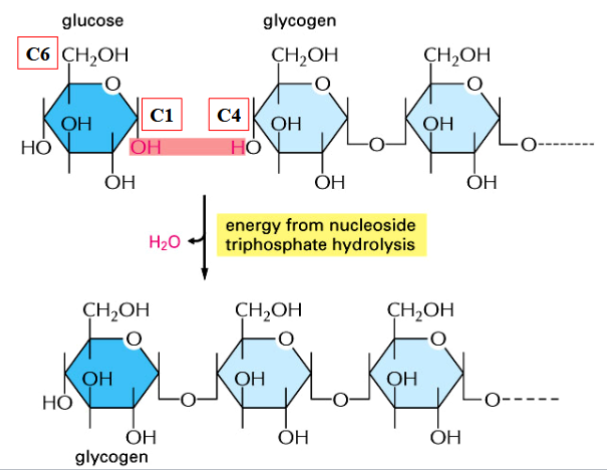
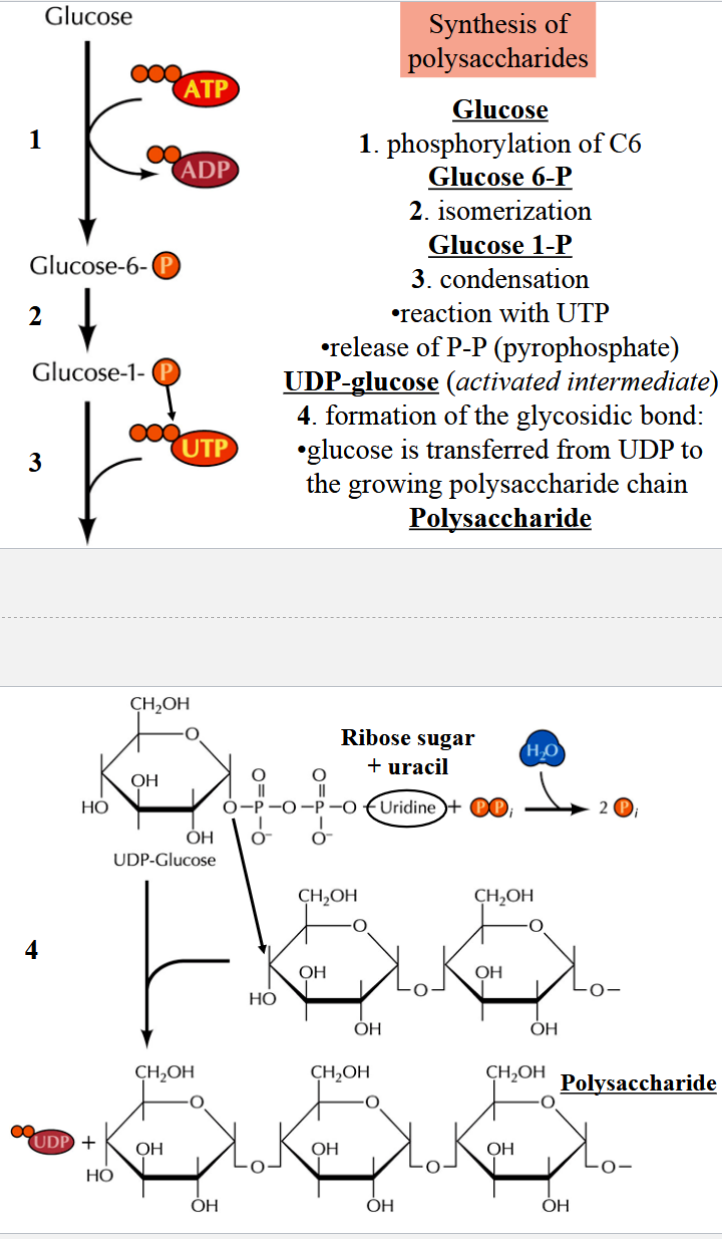
steps of polysaccharide synthesis
1) phosphorylation: glucose → glucose 6-P
2) isomerization: glucose 6-P → glucose 1-P
3) condensation: glucose 1-P → UDP-glucose (reaction with UTP and release of P-P)
(note: UDP-glucose is the activated intermediate)
4) formation of glycosidic bond: glucose is transferred from UDP to growing polysaccharide chain
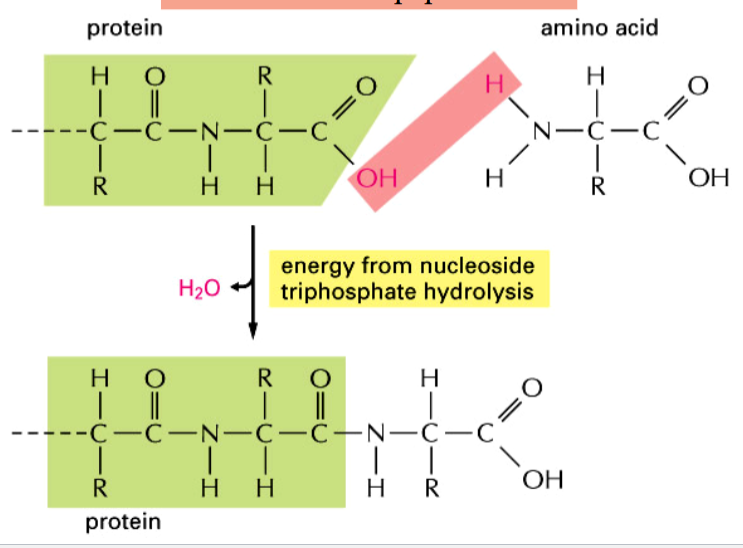
peptide bond formation
forms between amino acids and a growing protein (polypeptide) chain
dehydration synthesis
requires energy from nucleoside triphosphate hydrolysis
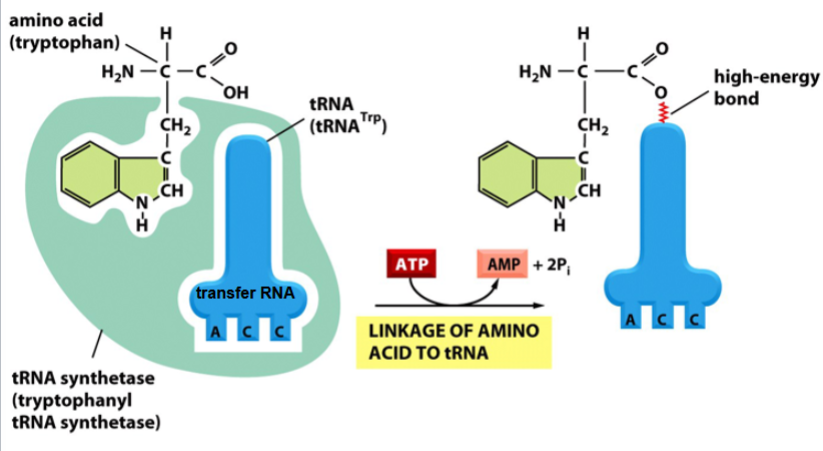
involve aminoacyl-tRNAs as an activated intermediate
aminoacyl-tRNAs
activated intermediates in the formation of a peptide bond
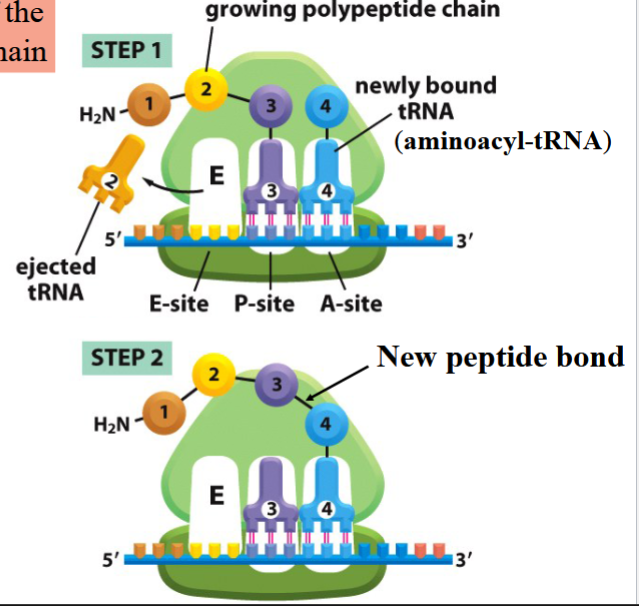
elongation of a polypeptide chain
3 sites: E, P, and A (eject/exit, present, and aminoacyl; a way to remember?)
a tRNA is ejected from the E site
P site has a growing polypeptide chain on it
A site has a newly-bound tRNA
bond forms between growing chain and amino acid on A site
tRNA on P shifts to E site, tRNA on A shifts to P site, a new aminoacyl-tRNA comes to A site
cycle repeats