CHEM 1150 EXAM 1 KEY
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Is there an attractive force between a pencil and the earth?
yes, it can be tested and answered through observations and experiments
Which of the following properties ascribed to atoms by Dalton do we still consider valid?
atoms of a given element are different from atoms of other elements
compounds are formed by combinations of atoms of two or more elements
Electrons are negatively charged. Which experiment provided evidence to support this claim? What is the specific evidence used to support this claim?
The cathode ray tube experiment, the beam of particles emitted form the cathode bent toward the positively charged plate
Which of the following contains atoms?
Dust
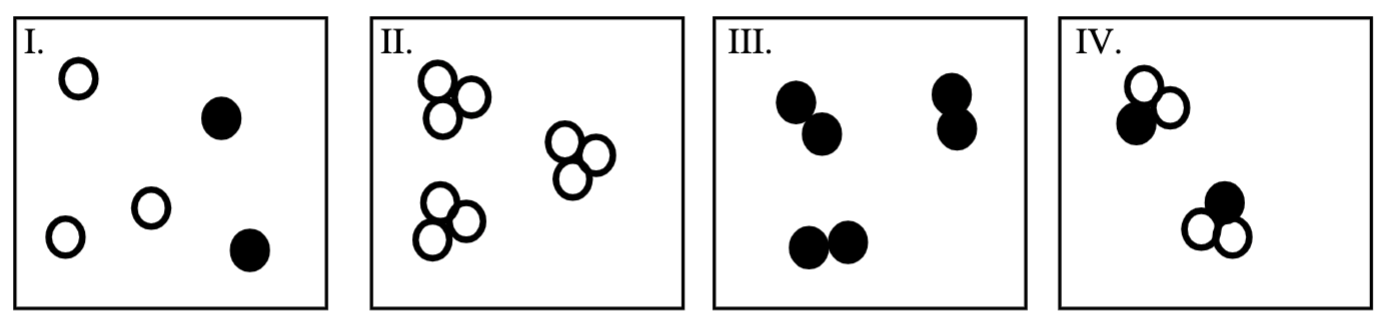
Which of the following represent molecules that are compounds?
IV only
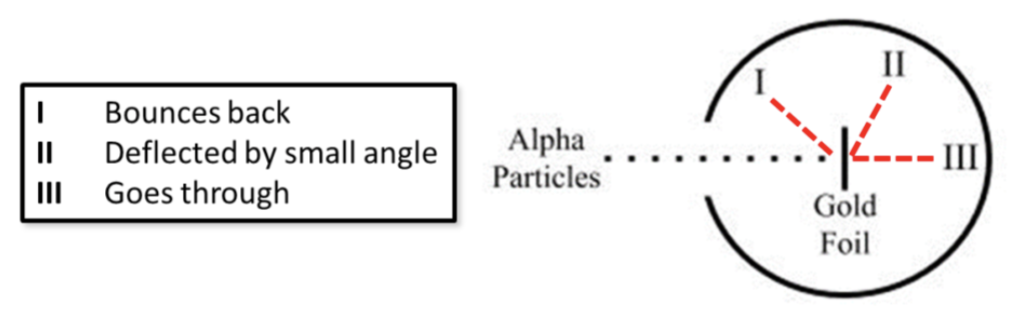
Where will the majority of the alpha particles be detected (I, II, or III) and why
III because a majority of the alpha particles go straight through the atom because it is mostly “empty” space
Thomson’s cathode ray tube experiment proved false which part of Dalton’s atomic theory?
All atoms are indivisible and indestructible
How would the strength of the electrostatic force change if the distance between two charged objects decreased?
The electrostatic force would become stronger
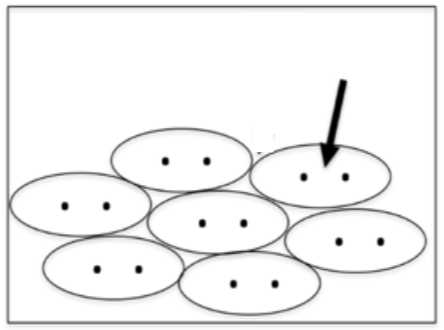
What does the arrow represent?
A covalent bond within a single molecule.
“A nitrogen molecule is made of the same type of atoms” Is this statement true or false and why?
true, because an element is made of one type of atom. Nitroden is an eleemnt that exists as diatomic molecules.
Why does the potential energy increase as two xenon atoms move extremely close together?
Because the repulsive force between the overlapping electron clouds is dominant
Which statement best describes the forces that exist between helium atoms in the solid state?
The partial positive end of one atom’s instantaneous dipole attracts the partial negative end of the neighboring atom’s induced dipole
When a covalent bond is formed between two nitrogen atoms:
Energy is released and a molecule is formed
The LDF between two neon atoms compared to the LDF between two argon atoms when both systems are the most stable is
weaker because neon has a smaller electron cloud, therefore there will be a smaller separation of charge, resulting in a weaker force
Which of the following are physical properties?
mass, color and density
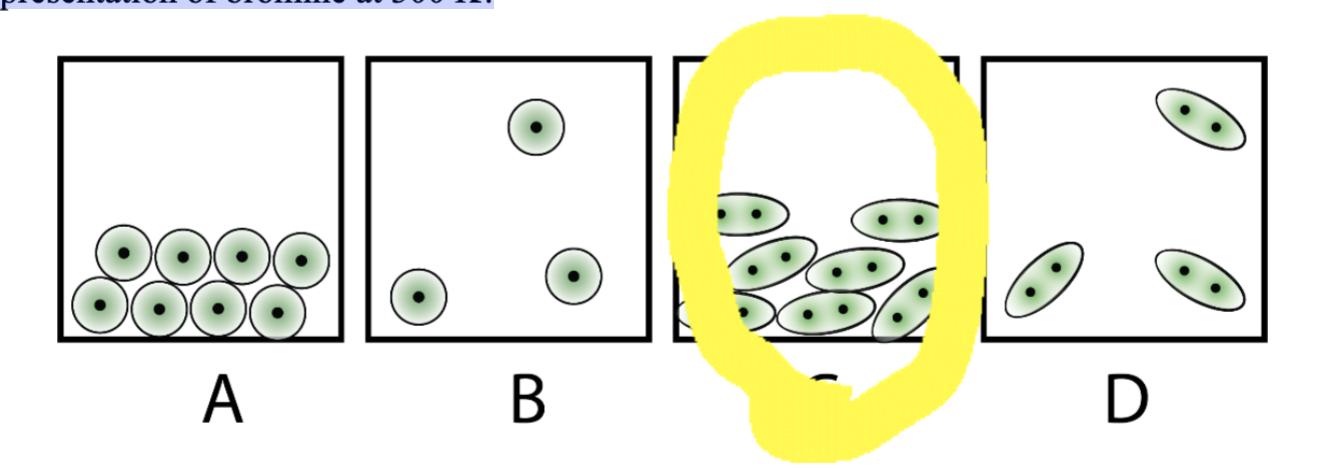
Bromine (Br2) melts at 266 K and boils at 332 K. Which of the following is the best
representation of bromine at 300 K?
What does the curve show? Which two subatomic particles could this curve represent?
The potential energy increases as the objects get closer together. One proton and one electron
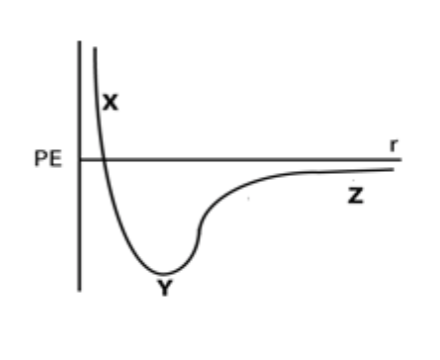
At very low temperatures, at which point in this curve would a system composed of two helium atoms be most stable?
Y, because the system has the lowest energy.
Which do you predict to have the higher boiling point, fluorine (F2) or neon (Ne) and why?
F2, because the LDFs between F2 molecules are stronger, requiring more energy to overcome.
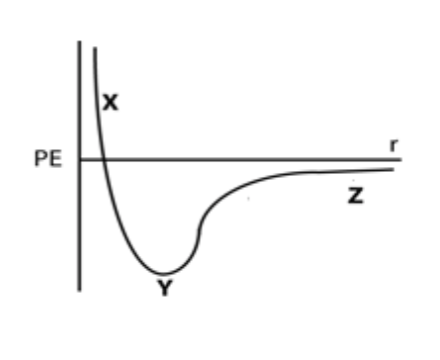
Consider a system of two atoms with a stable interaction between them (the atoms are stuck in the bottom of the potential energy well). The potential energy curve for this system is shown to the right. A third atom with higher KE is introduced form the surroundings. What would happen to the system for the potential energy to change from Y to Z?
I. The third atom from the surroundings would collide with the
system of two atoms.
II. Energy would transfer from the third atom (surroundings) to the system (original two atoms).
III. Energy would transfer from the system (original 2 atoms) to the third atom (surroundings)
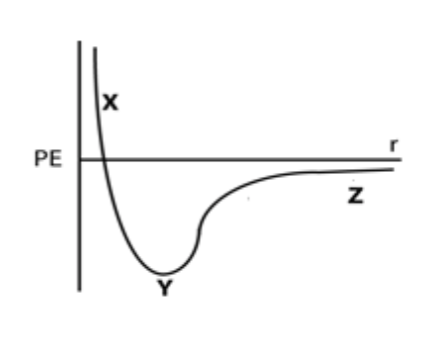
Use the potential energy curve in question 20 to explain the change in potential energy as two atoms move from Z to Y.
The potential energy becomes more negative because attractive forces between protons in one atom and electrons in the other become stronger
If the solid line in the potential energy curve below represents Xe, which dotted curve would represent He?
The one that looks the exact same but tinier.
Explain your reasoning from 22
He atoms are smaller in size and Xe atoms form stronger interactions than He atoms.
Which statement is closest to describing the first law of thermodynamics
conservation of energy
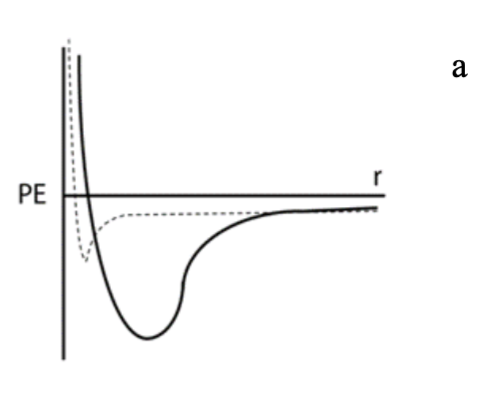
WHich curve represents a covalent bond?
The solid line because it takes more energy to separate the atoms.