C2.6 Giant covalent structures
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
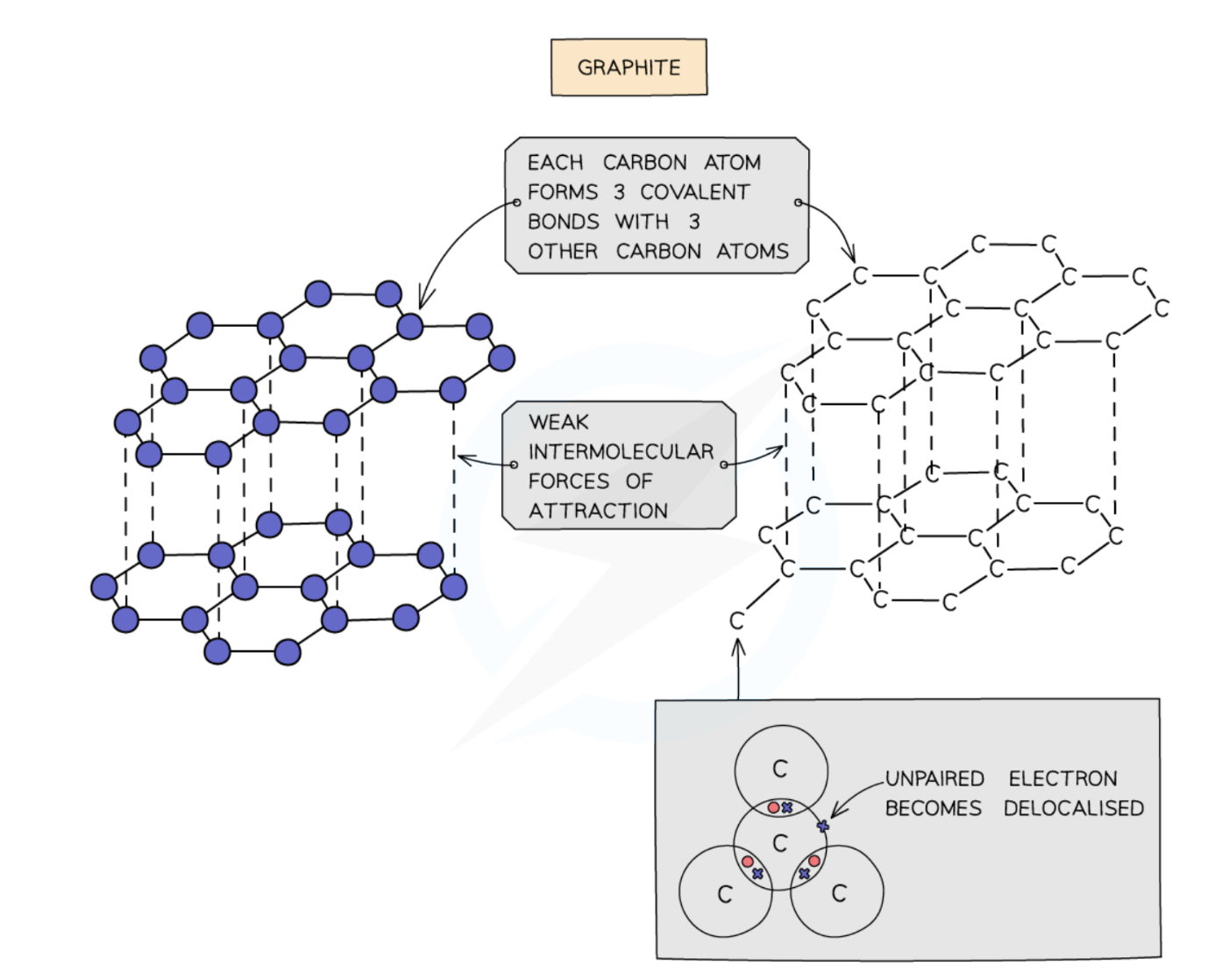
Describe the giant covalent structure of graphite
Each carbon atom is bonded to 3 other carbon atoms, leaving 1 free, delocalised electron per carbon atom
It can conduct electricity
Arranged in layers which can slide over each other because they are joined by weak intermolecular forces, but the covalent bonds between carbon atoms are strong
High melting and boiling point
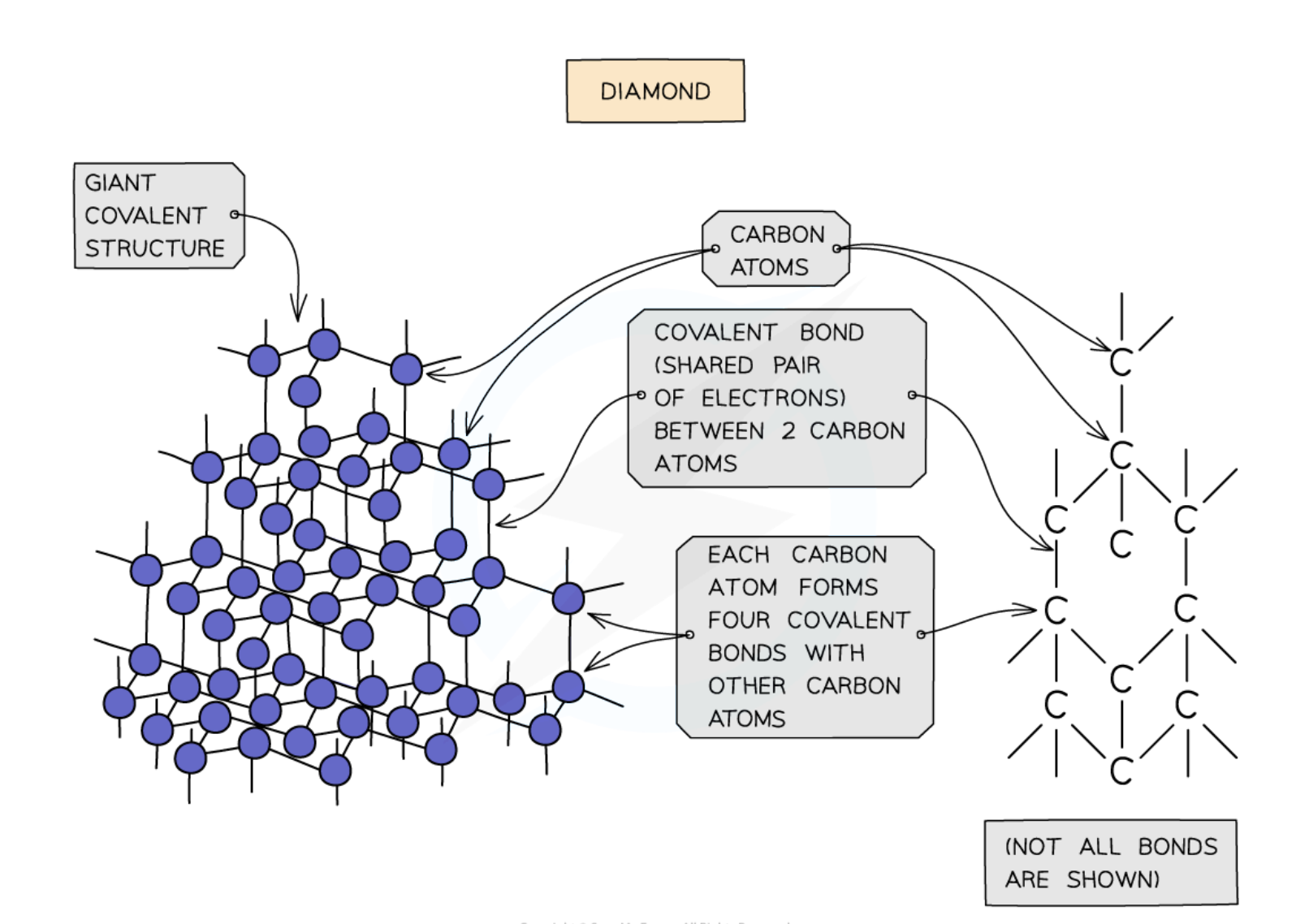
Describe the giant covalent structure of diamond
Each carbon atom is bonded to 4 other carbon atoms
No free electrons = cannot conduct electricity
No intermolecular forces, very strong covalent bonds
Hard
High melting and boiling point
Relate the structure and bonding of graphite to its uses
Uses: lubricant and electrode
Lubricant: weak intermolecular forces between layers allow carbon atoms to slide over each other, making it very slippery and smooth
Electrode: good electrical conductor since it has delocalised ions which can move and carry an electrical charge
Relate the structure and bonding of diamond to its uses
Uses: cutting tools
Cutting tools: diamond is extremely hard and dense, making it an excellent cutting tool