MCDB 310 Lecture 7
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
63 Terms
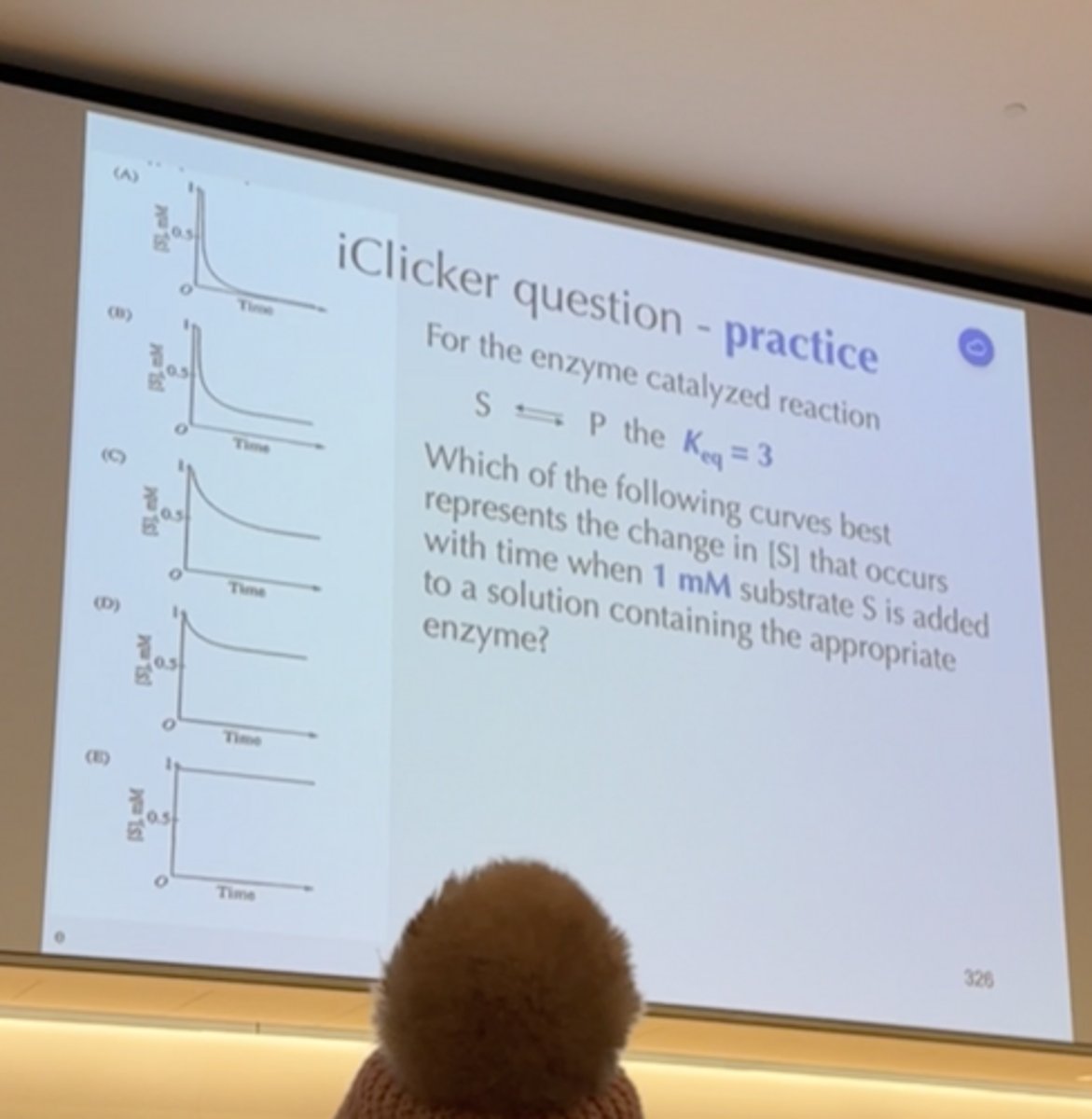
Iclicker
E
Kinetics
is the study of the rate at which
compounds react
Rate of enzymatic reaction is affected by
– Enzyme
– Substrate
– Effectors
– Temperature
Why do we care about the reaction speed of a enzyme reaction
• Quantitative description of biocatalysis
• Determine the order of binding of substrates
• Understand catalytic mechanism
• Find effective inhibitors
• Understand regulation of activity
How do we perform kinetic measurements? (experiment)
1) Mix enzyme + substrate
2) Record rate of product formation as a function of time (the velocity of reaction)
3) V0 is the velocity at time ≈ 0
4) Change substrate concentration and determine new V0 (you run out of substrate at platu)
5) Plot initial velocity (V0) versus substrate concentration [S]
![<p>1) Mix enzyme + substrate</p><p>2) Record rate of product formation as a function of time (the velocity of reaction)</p><p>3) V0 is the velocity at time ≈ 0</p><p>4) Change substrate concentration and determine new V0 (you run out of substrate at platu)</p><p>5) Plot initial velocity (V0) versus substrate concentration [S]</p>](https://knowt-user-attachments.s3.amazonaws.com/d7e36a98-9915-46f2-9fff-5f999cf3504b.jpg)
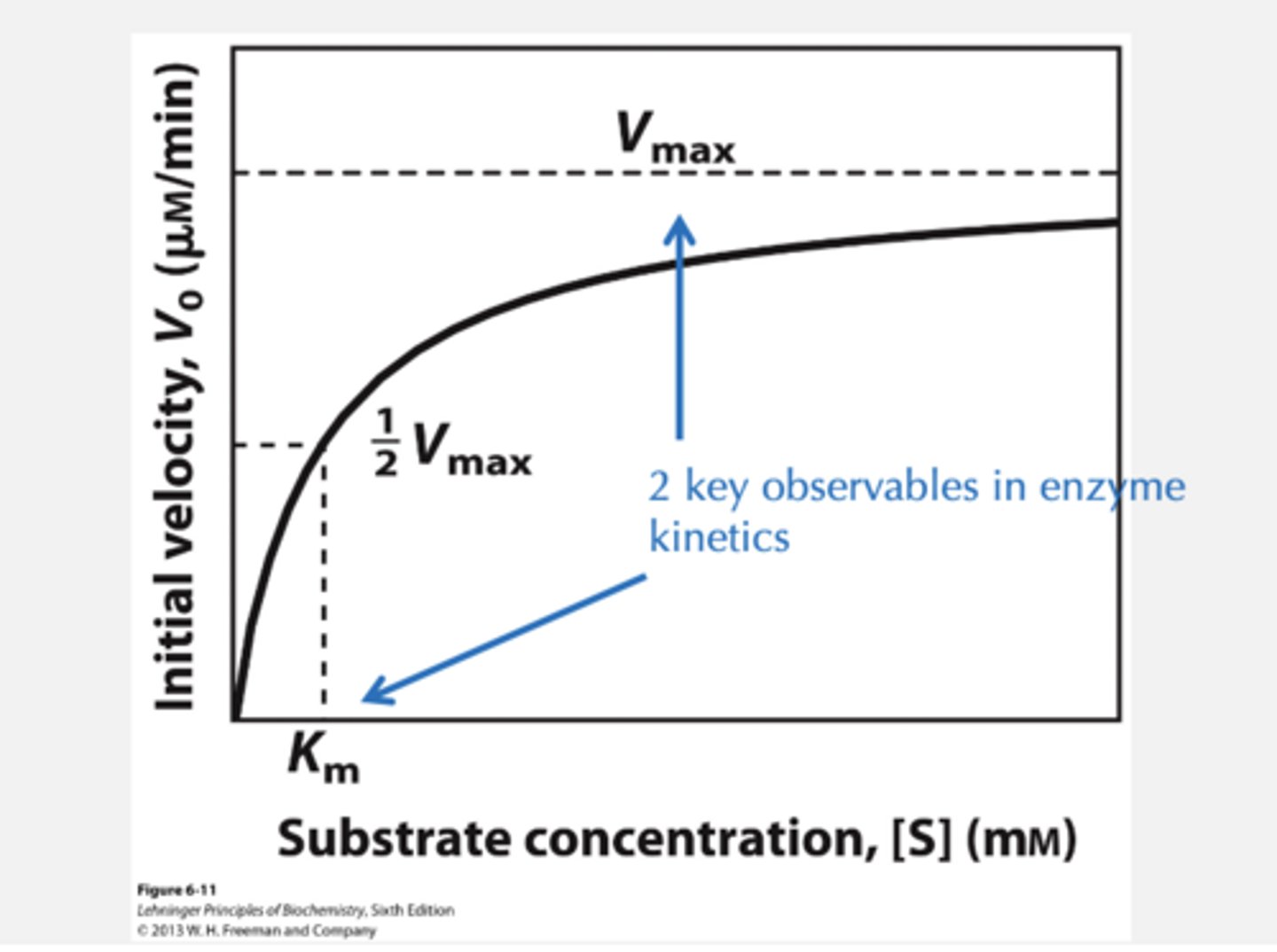
Michaelis-Menten Kinetics/ plot
Plot initial rate against substrate
concentration
The beginnings of enzymology
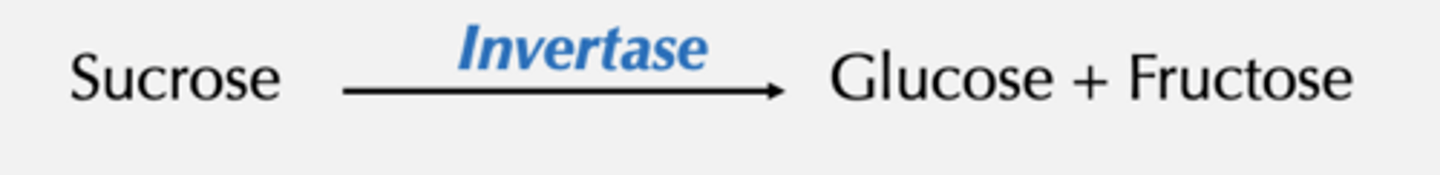
Observation of Adrian Brown (1902)
At low concentrations of sucrose, reaction rate was directly dependent on the sucrose concentration
Example: k = 0.1 s-1 : 10% of the substrate reacts within 1 sec

When When [sucrose] was very high
reaction rate reached maximum (Vmax) and was no longer dependent on [sucrose]
Conclusion of Brown observations
• The reaction must be composed of two individual reactions
• One reaction is rate limiting at low [S] and the other at high [S]
The Michaelis-Menten Model
• Enzyme and substrate form an enzyme/substrate complex, ES
• ES can either dissociate or substrate is converted into product P
• P dissociates and free enzyme is regenerated

Low [S] vs High [S] what happens in the Michaelis-Menten Model
Low [S] -> formation of [ES] is rate limiting -> rate depends directly on [S]
High [S] -> all binding sites of E bound by S -> E is permanently in [ES] complex (adding more S makes no
difference) -> Breakdown of ES to E + P becomes rate limiting step
Saturation Kinetics: The initial and latter stages of MM kinetics
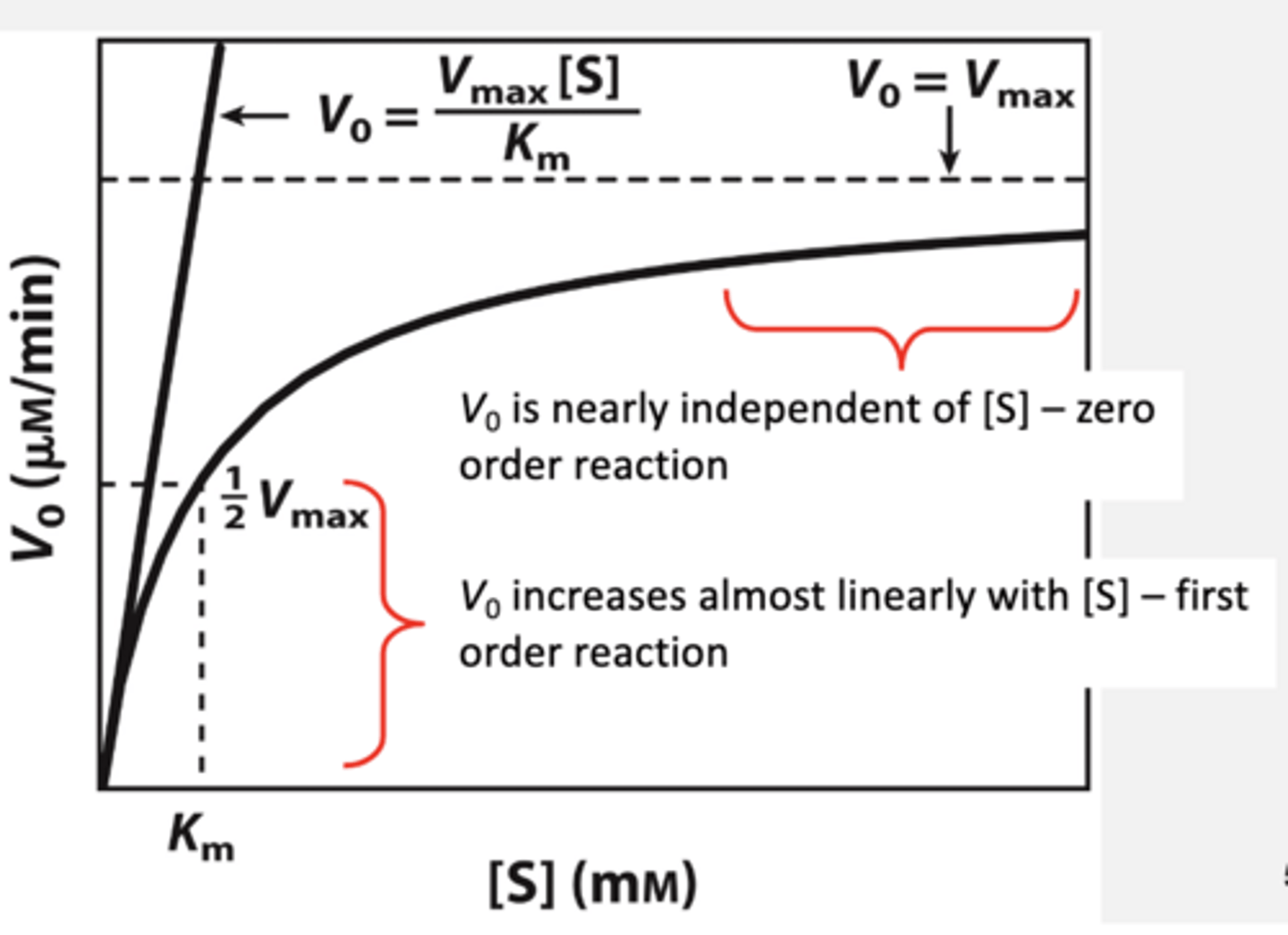
MichaelisConstant KM
If [S] = KM
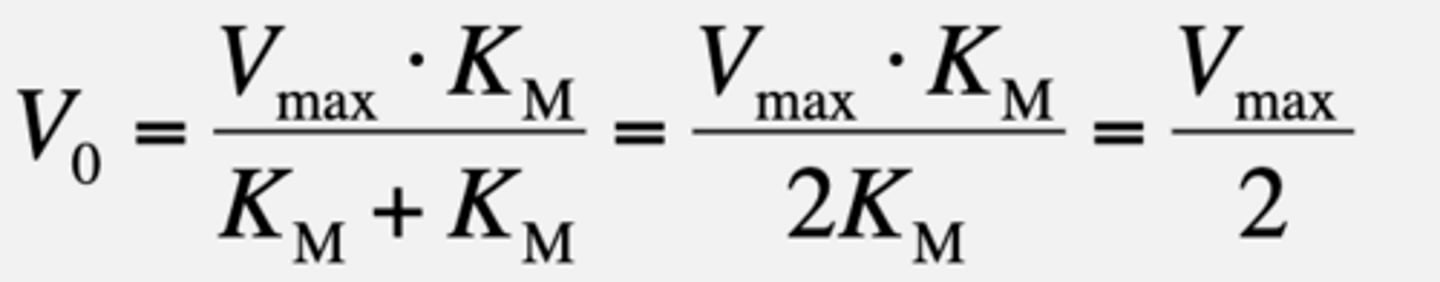
KM is the substrate concentration at which the ----.
velocity of the reaction is half maximal
What does a low Km mean
• Low KM -> maximum velocity achieved at lower [S]
but it doesn't mean anything if you are at a very high S concentration
Definition of KM and what K-1>> Kcat
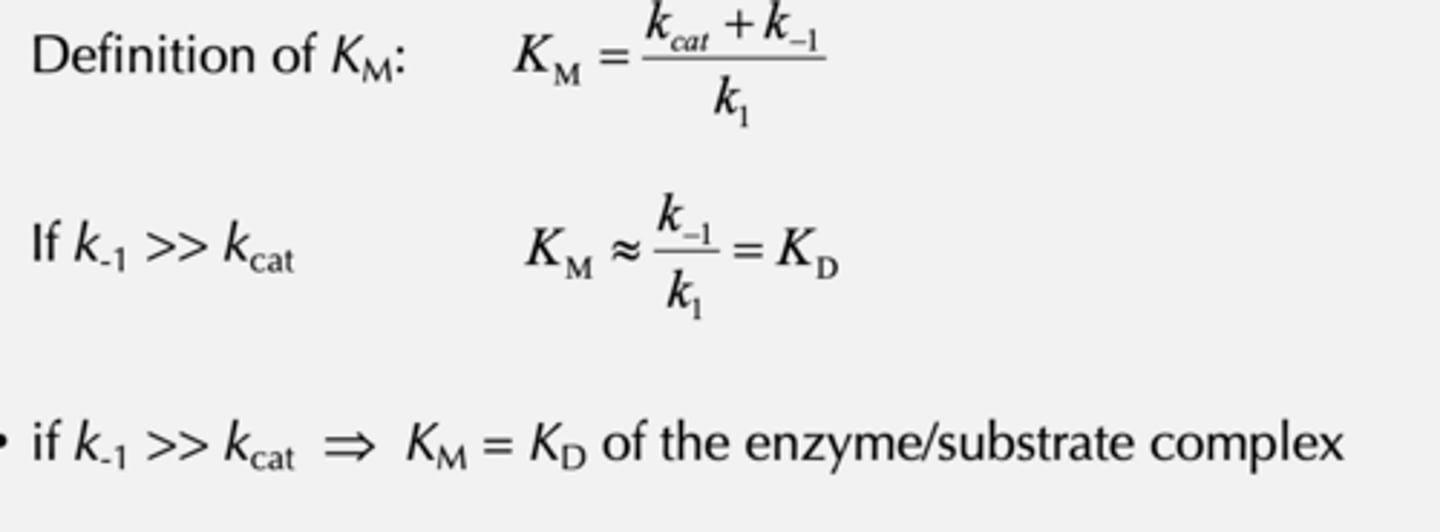
KM gives an estimate for what
KM gives an estimate for the KD of the enzyme for its substrate
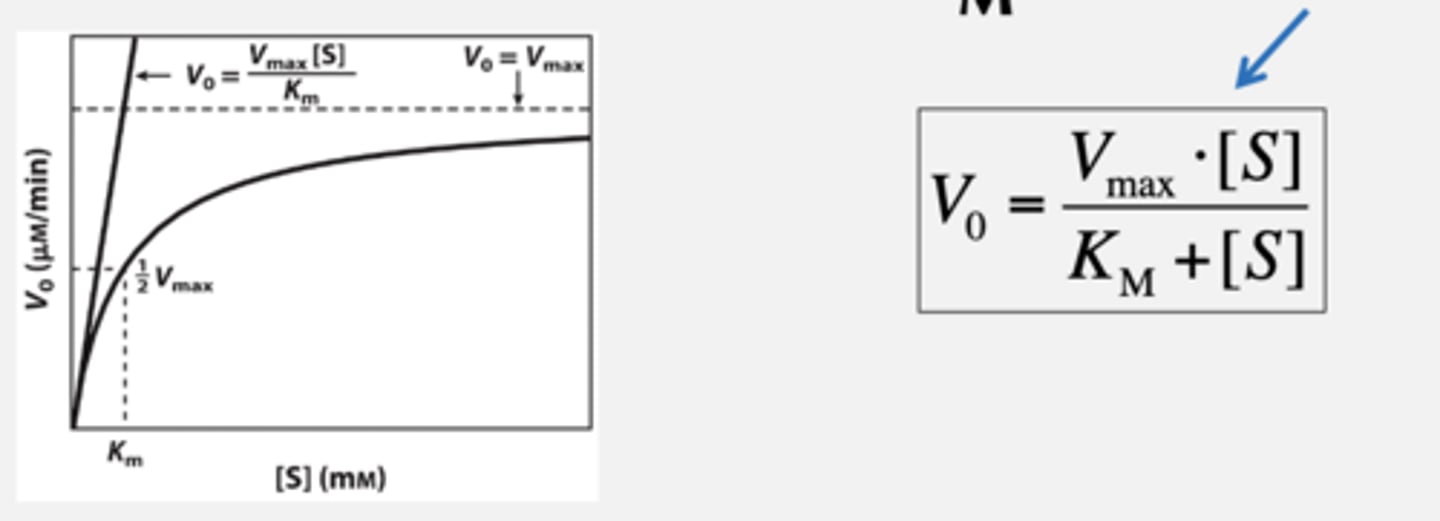
What does Vmax tell us? Equation and Vo and Vmax equation.
Maximal rate of a reaction when enzyme is fully saturated
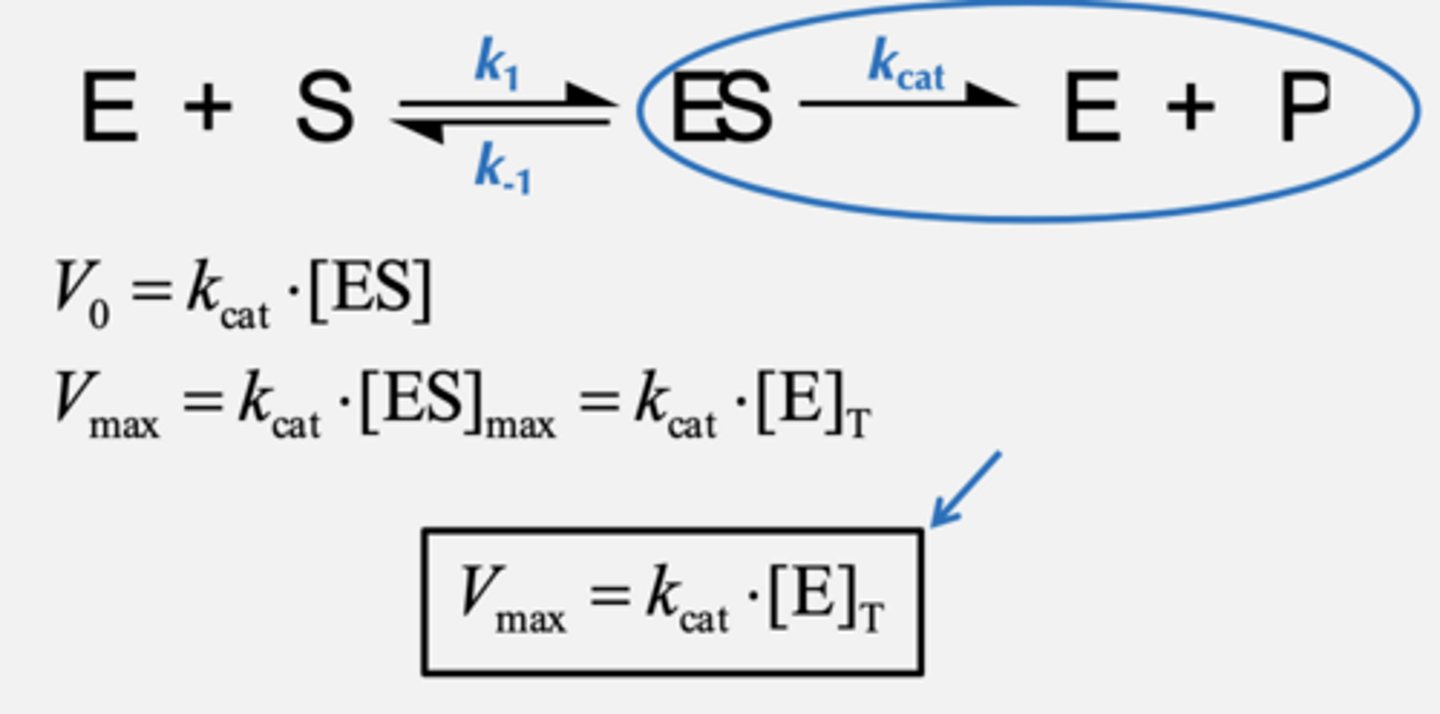
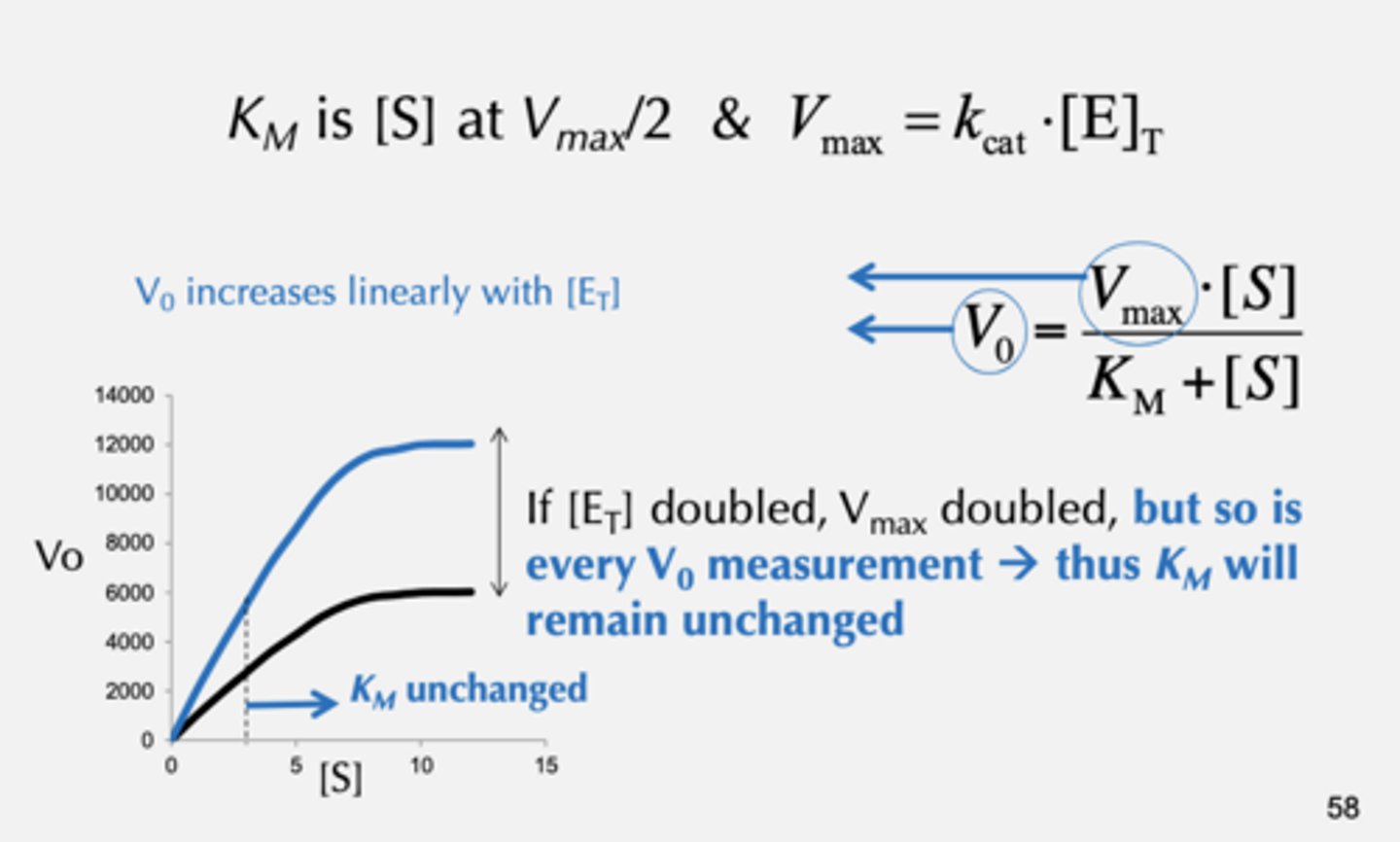
When Does Vmax for a Given Enzyme Change?
Vmax is directly proportional to [E]T
Does kcat depend on enzyme concentration?
Does KM depend on enzyme concentration?
No
KM and kcat are independent of [E]T
Rate constants are independent of concentration terms
![<p>No</p><p>KM and kcat are independent of [E]T</p><p>Rate constants are independent of concentration terms</p>](https://knowt-user-attachments.s3.amazonaws.com/aa988bdc-0c29-49b8-9a13-339bbd330765.jpg)
But what about the dependence
of KMà Vmax -> [E]T???
Catalytic Constant kcat (turnover number)
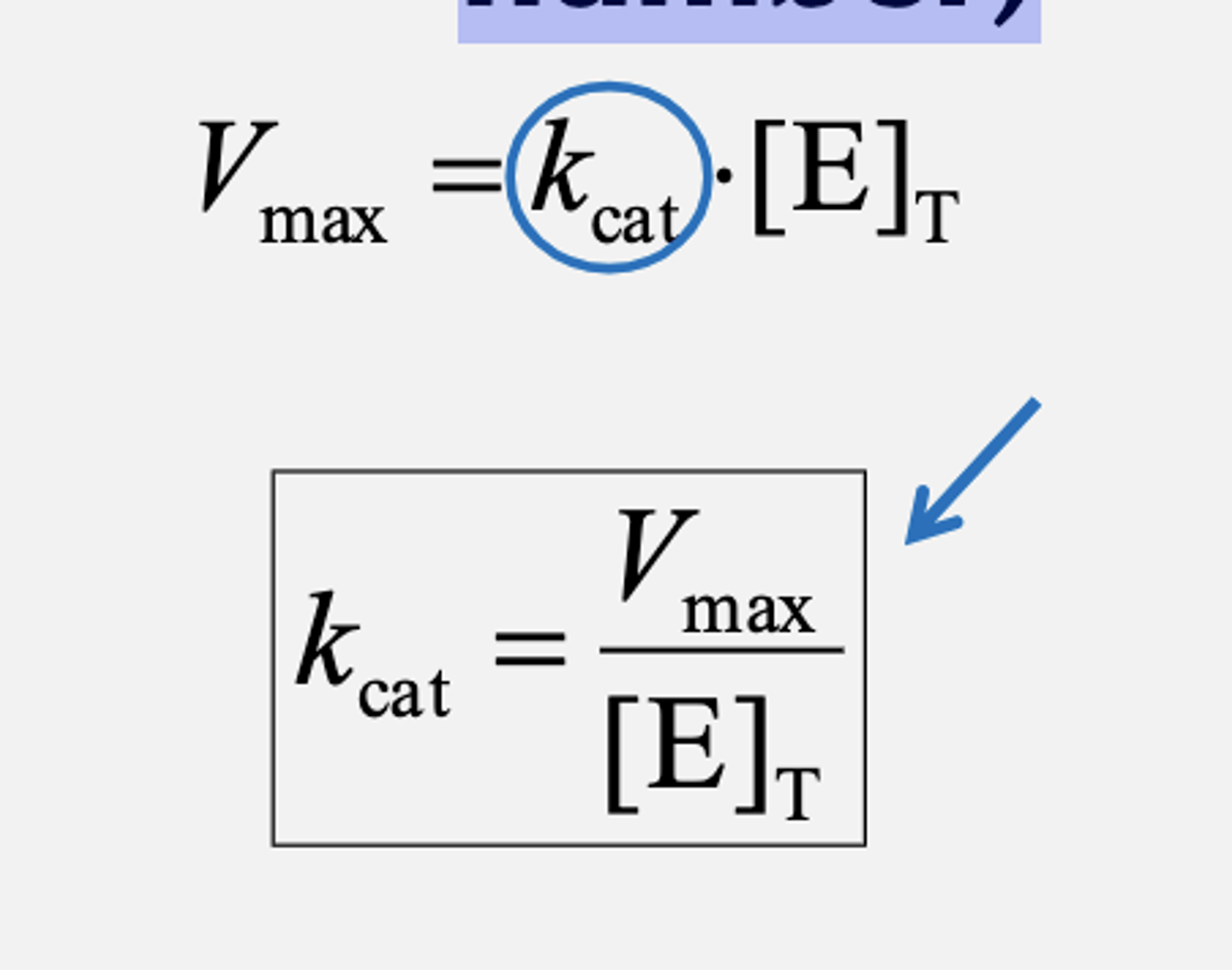
kcat
Turn-over number of the enzyme, i.e. how many substrate molecules are converted to product per second
by one enzyme molecule under conditions of saturation
How fast an enzyme catalyzes a reaction when it
encounters its substrate
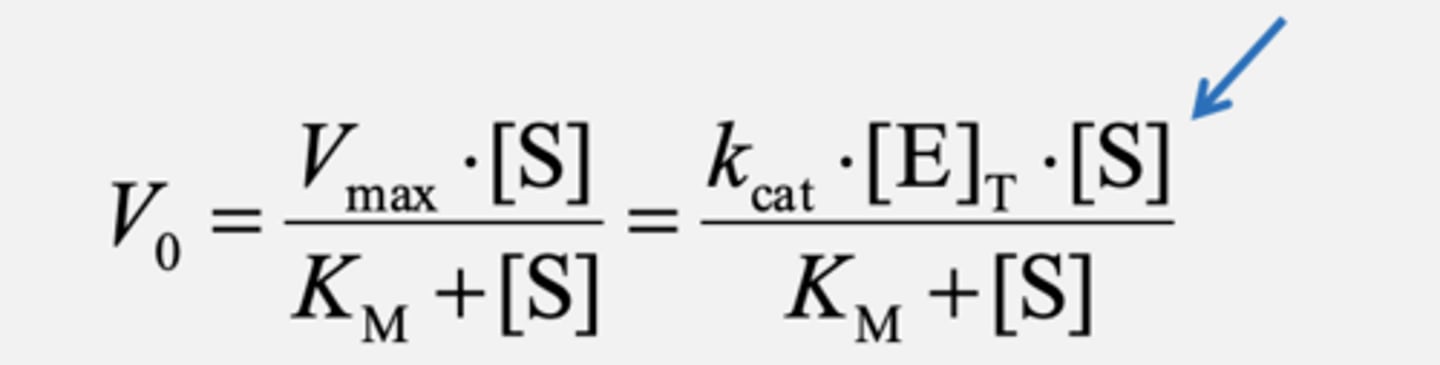
If [S] << KM
Second-order reaction with kcat/KM being the second-order rate constant!
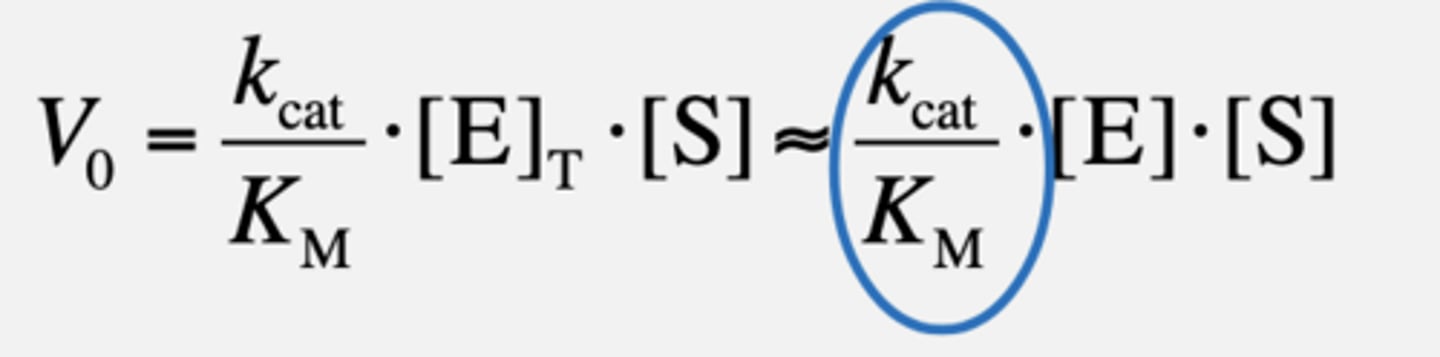
kcat/KM - Catalytic Efficiency
• Cannot be faster than frequency with which E and S collide with each other
in solution
• Upper limit for kcat/KM is diffusion (108-109 M-1 s-1)
Enzymes with such kcat/KM values (upper limit) have achieved
"catalytic perfection"
-Reaction is catalyzed almost every time enzyme encounters a substrate
molecule
Enzyme efficiency is limited by diffusion: kcat/KM. How can it gain efficiency
by having high kcat or low KM or both - Catalase vs. acetylcholinesterase
Recap slide
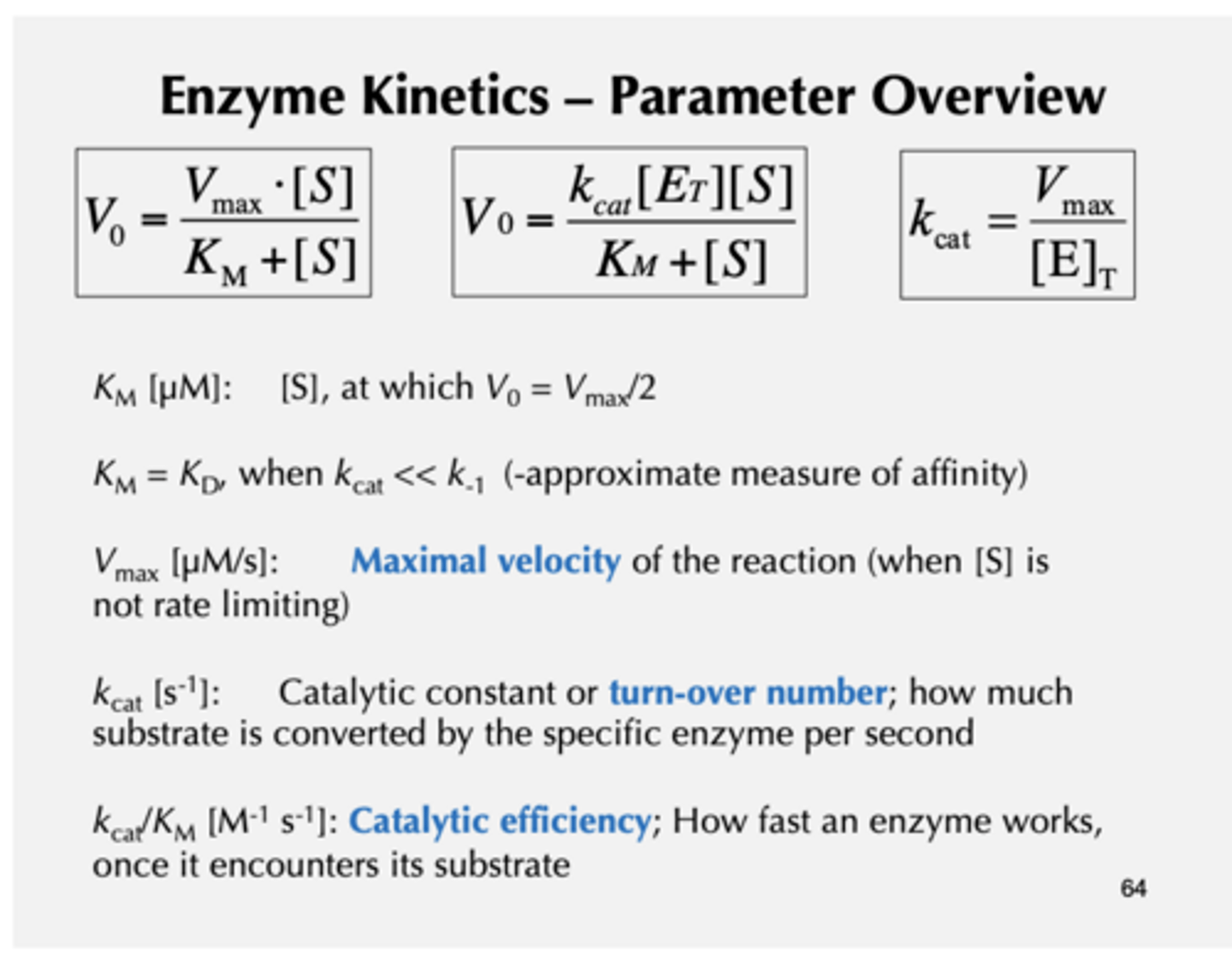
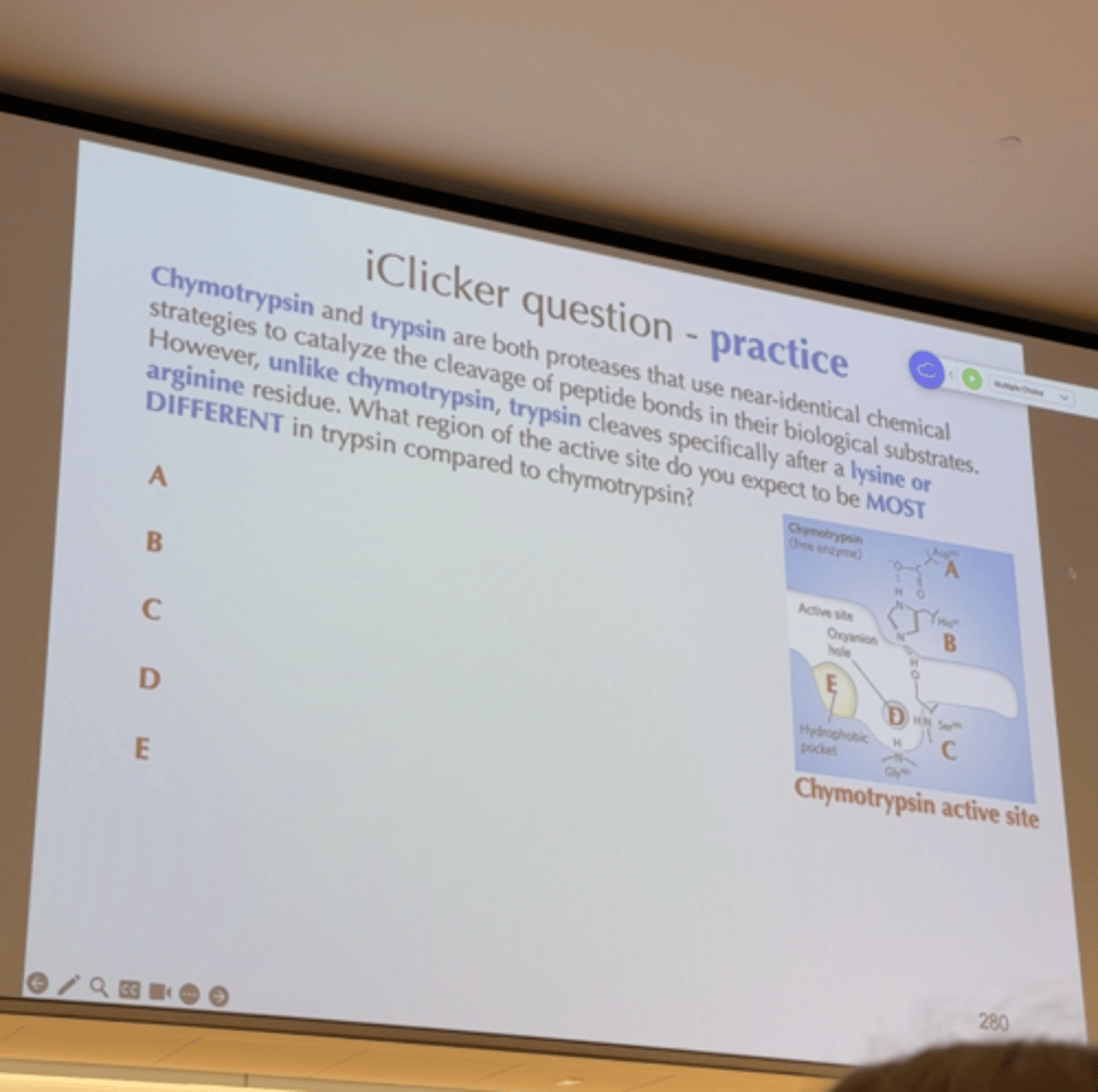
iclicker
A
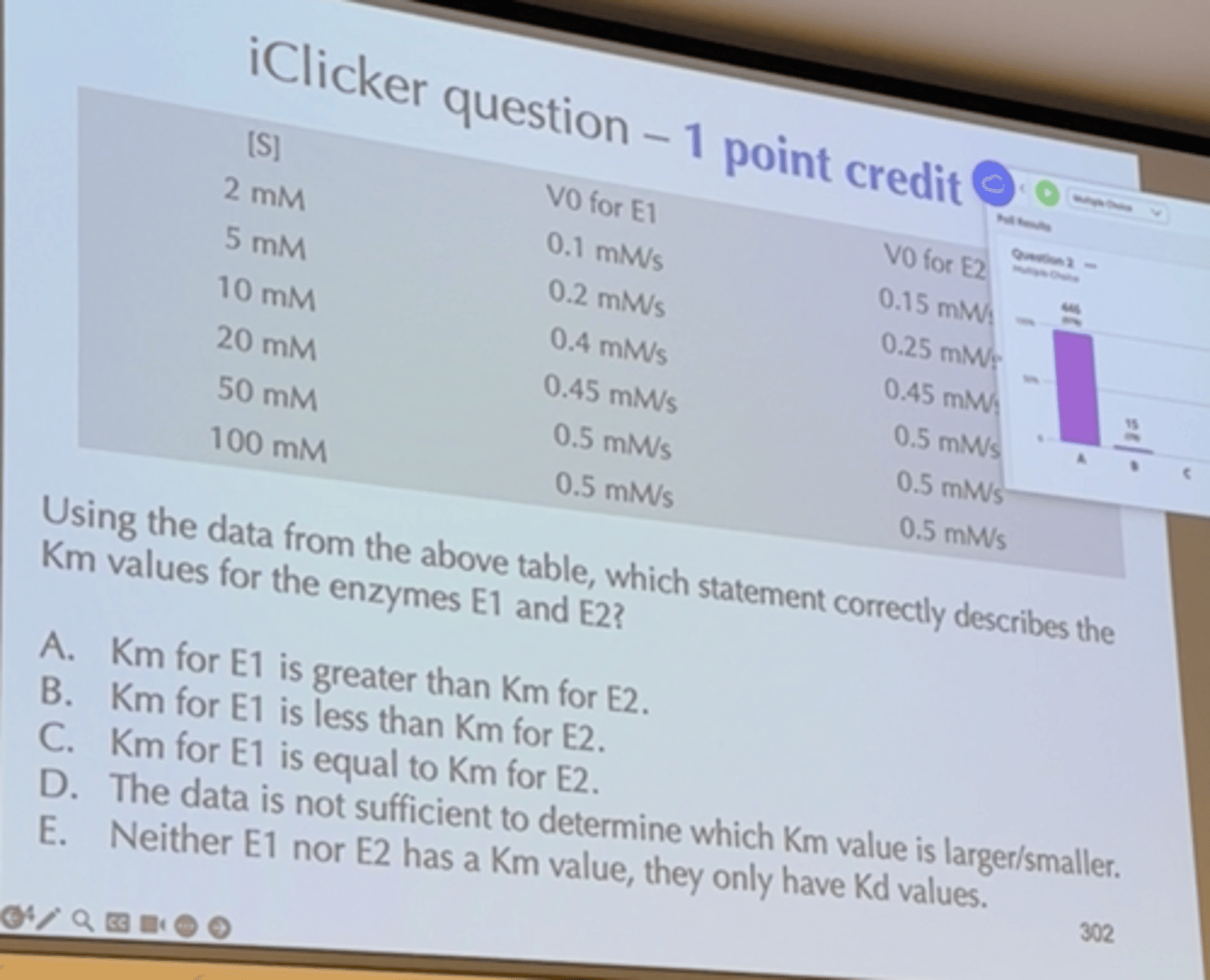
Determination of Kinetic Parameters
Nonlinear Michaelis-Menten plot should be used to calculate parameters Km and Vmax
Linearized double-reciprocal plot is good for analysis of two-substrate data or inhibition
Lineweaver-Burk Plot
Linearized, Double-Reciprocal
1. Determine Vmax from y intercept
2. Determine KM from x intercept
Enzyme Inhibition
inhibitors are compounds that decrease enzyme's activity
Reversible inhibitors and what they can bind?
bind to and can dissociate from the enzyme
• They are often structural analogs of substrates or products
• They are often used as drugs to slow down a specific enzyme
They can bind:
• to the free enzyme and prevent the binding of the substrate
• to the enzyme-substrate complex and prevent the reaction
Irreversible inhibitors (inactivators)
react with the enzyme
• One inhibitor molecule can permanently shut off one enzyme molecule
• They are often powerful toxins but also may be used as drugs
What you need to know about inhibitors
• The mechanism (what do they bind: E or ES or both?)
• How will the Lineweaver Burk plot change with [I] for each kind of inhibition mechanism
• If you are given the plot, you should be able to identify what kind of inhibition mechanism it is
• What happens to KM and Vmax for each kind of mechanism
• You should know if a particular inhibition mechanism works at low [S] or high [S]
Competitive Inhibition
Inhibitor binds at the same site as the substrate and competes with it
• Competitive inhibitor acts by reducing the free concentration of enzyme available for substrate binding
when is Competitive Inhibition effective
• Inhibitor is effective at low [S]
Increasing [S] will outcompete inhibitor (so, is Km or Vmax affected?)
You Km will increase
Vmax will stay the same
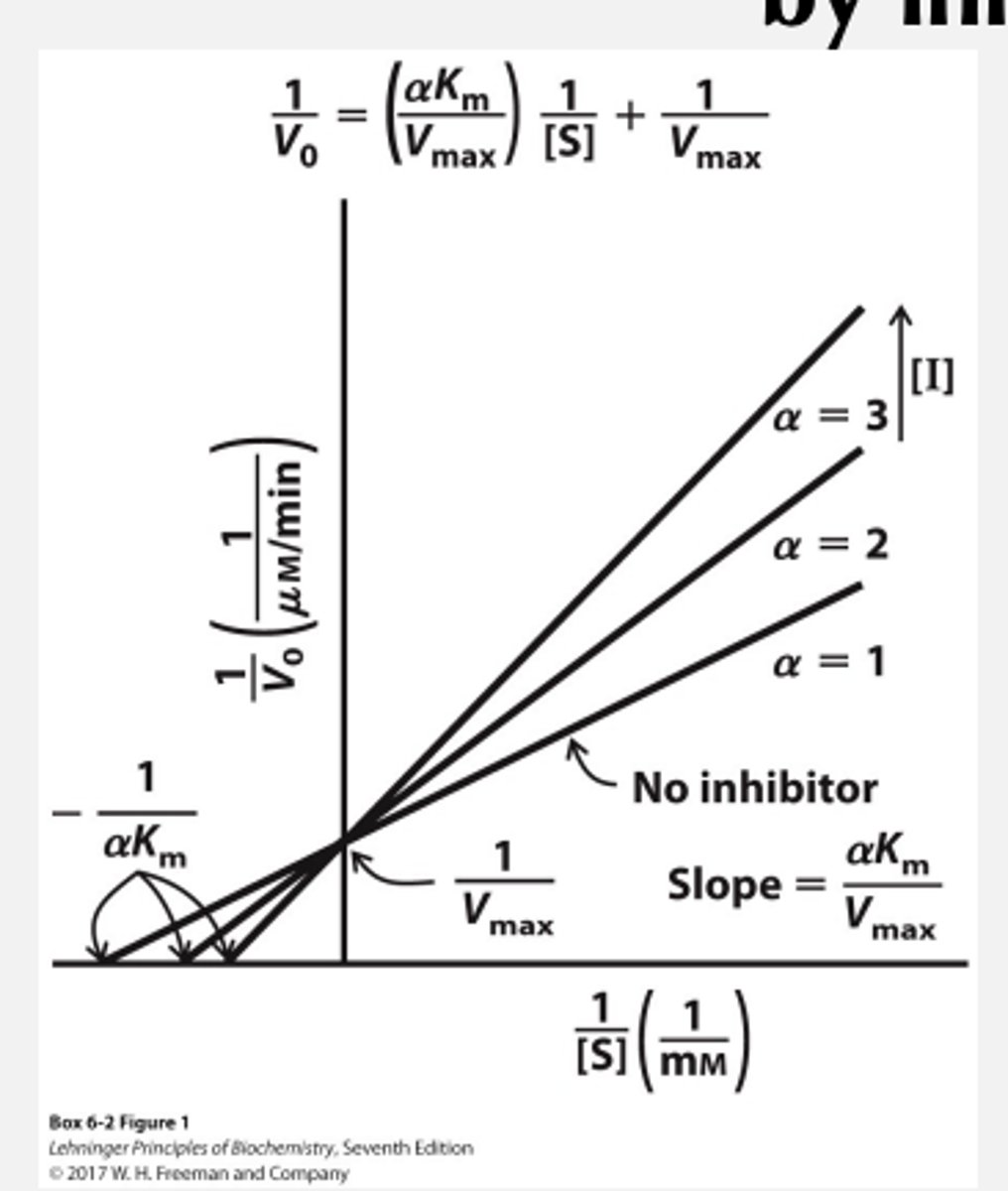
Inhibitor dissociation constant. Along with alpha or how well competitive inhibitor works
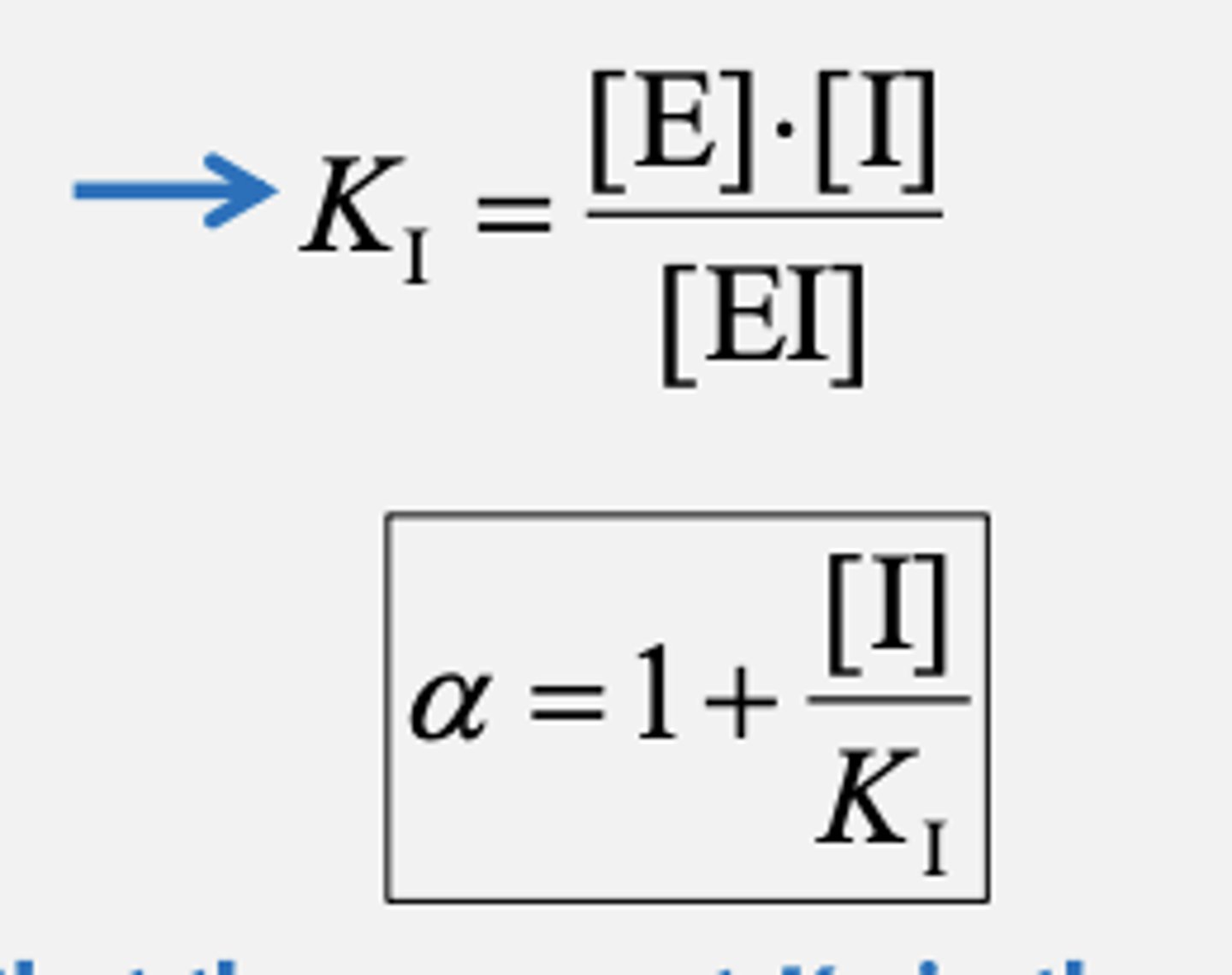
by what factor does Km increase and what does this mean?
KM increases by a factor of a, which means that the apparent KM in the presence of the inhibitor will be a x KM
Uncompetitive Inhibition
Inhibitor binds to the ES complex but not to the free enzyme and affects the maximal activity of the enzyme
•Does not need to resemble substrate
What does Uncompetitive Inhibition cause? When is it the most effective?
•Causes structural distortion of the active site without affecting substrate binding
•Inhibitor binding distorts the ES complex, which inhibits catalysis
•Most effective at high [S]
![<p>•Causes structural distortion of the active site without affecting substrate binding</p><p>•Inhibitor binding distorts the ES complex, which inhibits catalysis</p><p>•Most effective at high [S]</p>](https://knowt-user-attachments.s3.amazonaws.com/6e932615-dfbb-4d67-8c6b-4989721dfeb0.png)
Inhibitor dissociation constant
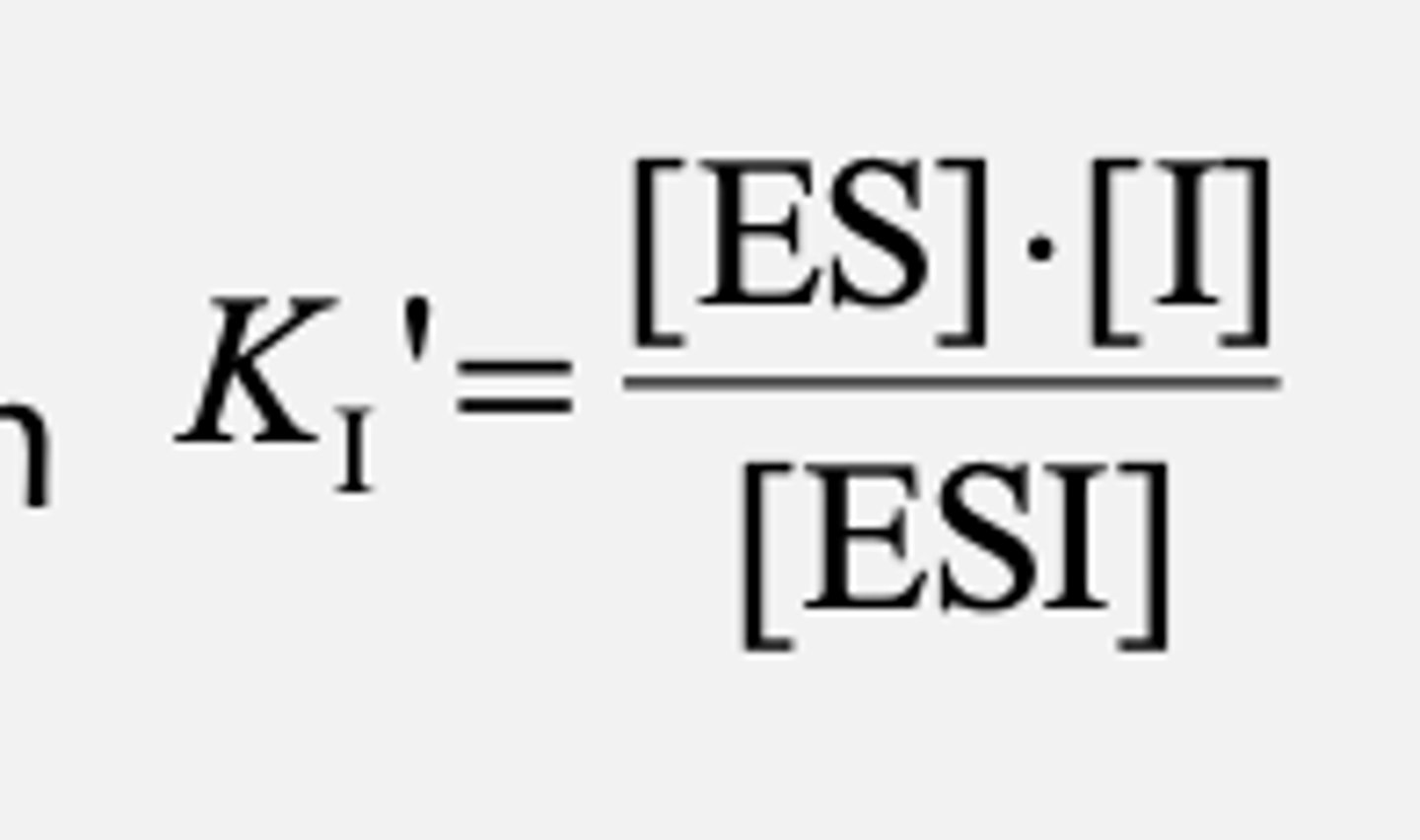
Uncompetitive Inhibition Vmax and Km
• Vmax is lowered
• Because I stabilizes ES, less substrate is needed to reach saturation -> KM is lowered
*See parallel it must be competitive
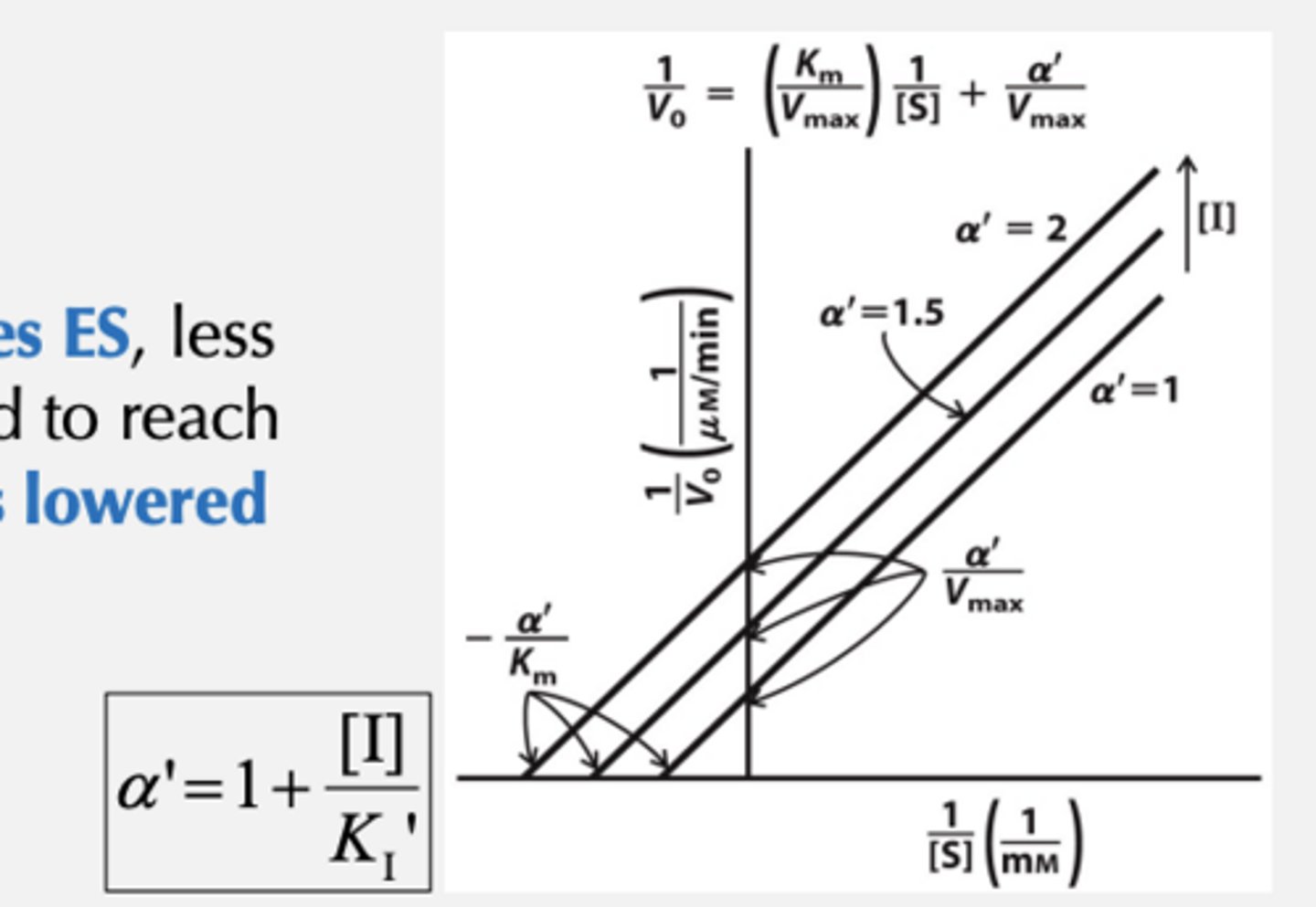
How much is Vmax and Km decreased by? What does this mean?
Both Vmax and KM decrease by a' and therefore the apparent Vmax in the presence of the inhibitor becomes Vmax/a' .The apparent KM in the presence of the inhibitor becomes KM/a'
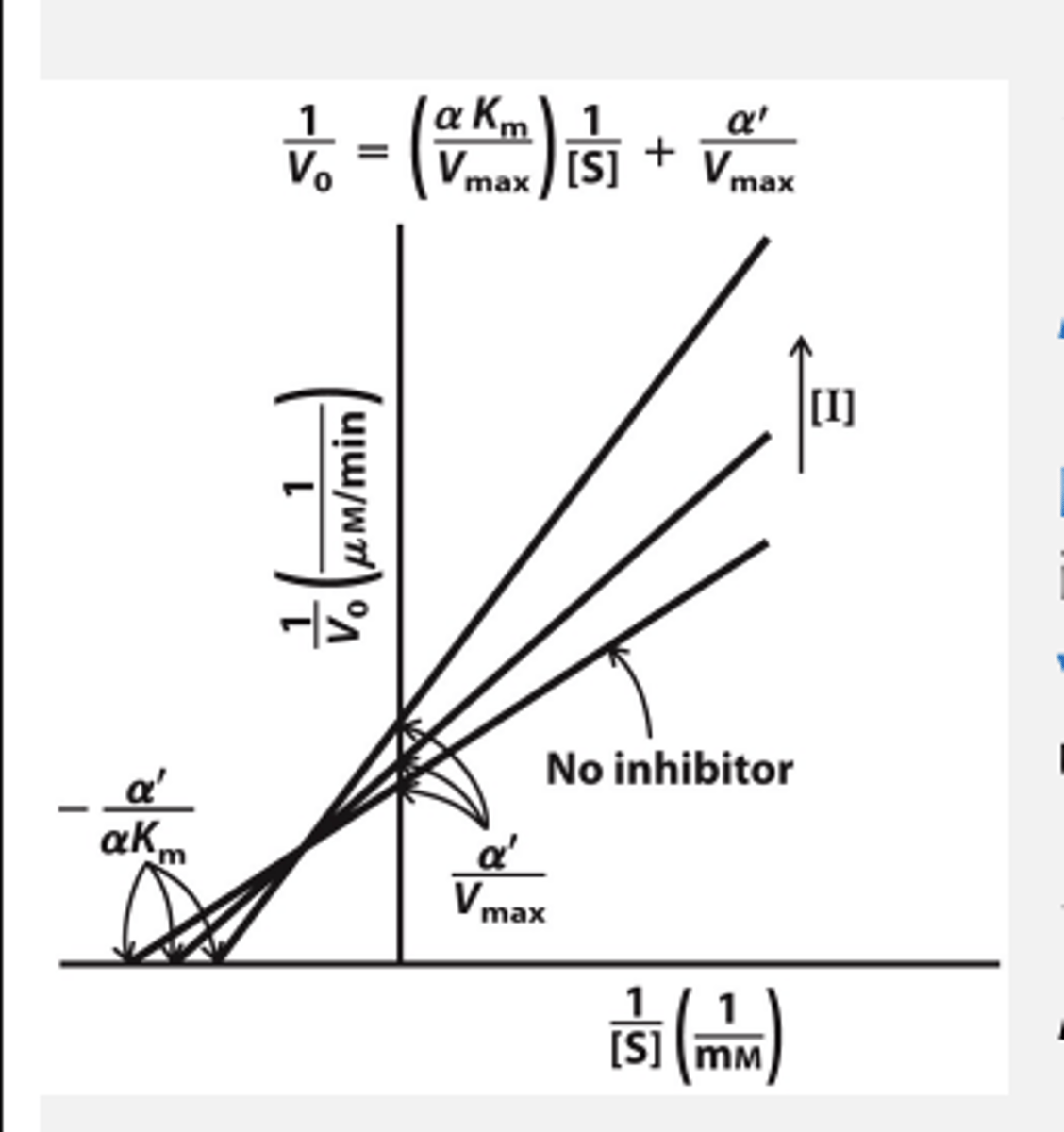
Mixed Inhibition
Inhibitor binds to both free enzyme and ES complex
- works at both low and high [S]
![<p>Inhibitor binds to both free enzyme and ES complex</p><p>- works at both low and high [S]</p>](https://knowt-user-attachments.s3.amazonaws.com/571eec22-300b-4e25-9d08-edb42ba8930e.png)
Mixed inhibition Vmax and Km
Vmax decreases (by a'). Apparent Vmax = Vmax /a'
KM is altered by a/a'. Apparent KM = KM x a/a'
Non-competitive is special case of mixed inhibition: when KI = KI ' à only Vmax reduced, but KM is unchanged
is special case of mixed inhibition
When KI = KI ' -> only Vmax reduced, but KM is unchanged
What does KI = KI ' mean?
Inhibitor binds E and ES with same affinity
Mixed Inhibition plot and example
Inhibitors of HIV1 Protease
iclicker
B
they both decrease

Noncovalent Modification: Allosteric Regulators
Allosteric regulators do not follow Michaelis-Menten kinetics
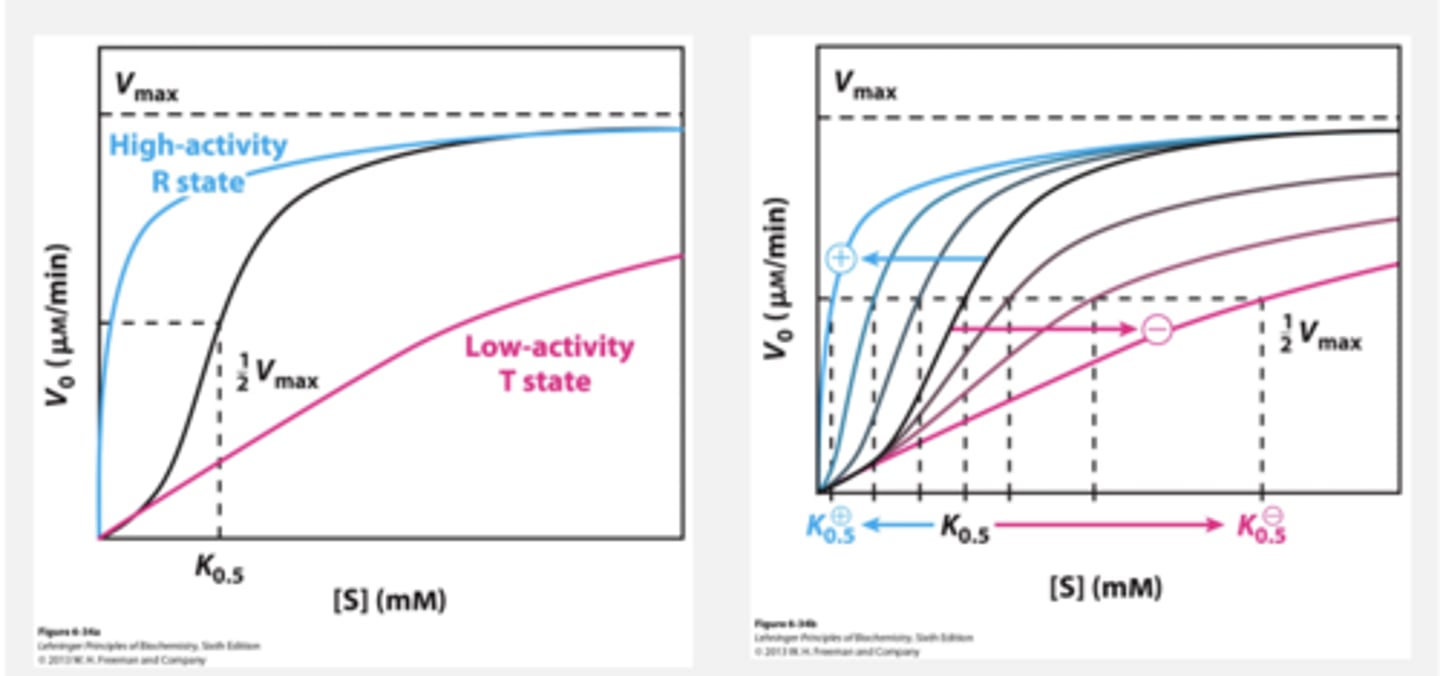
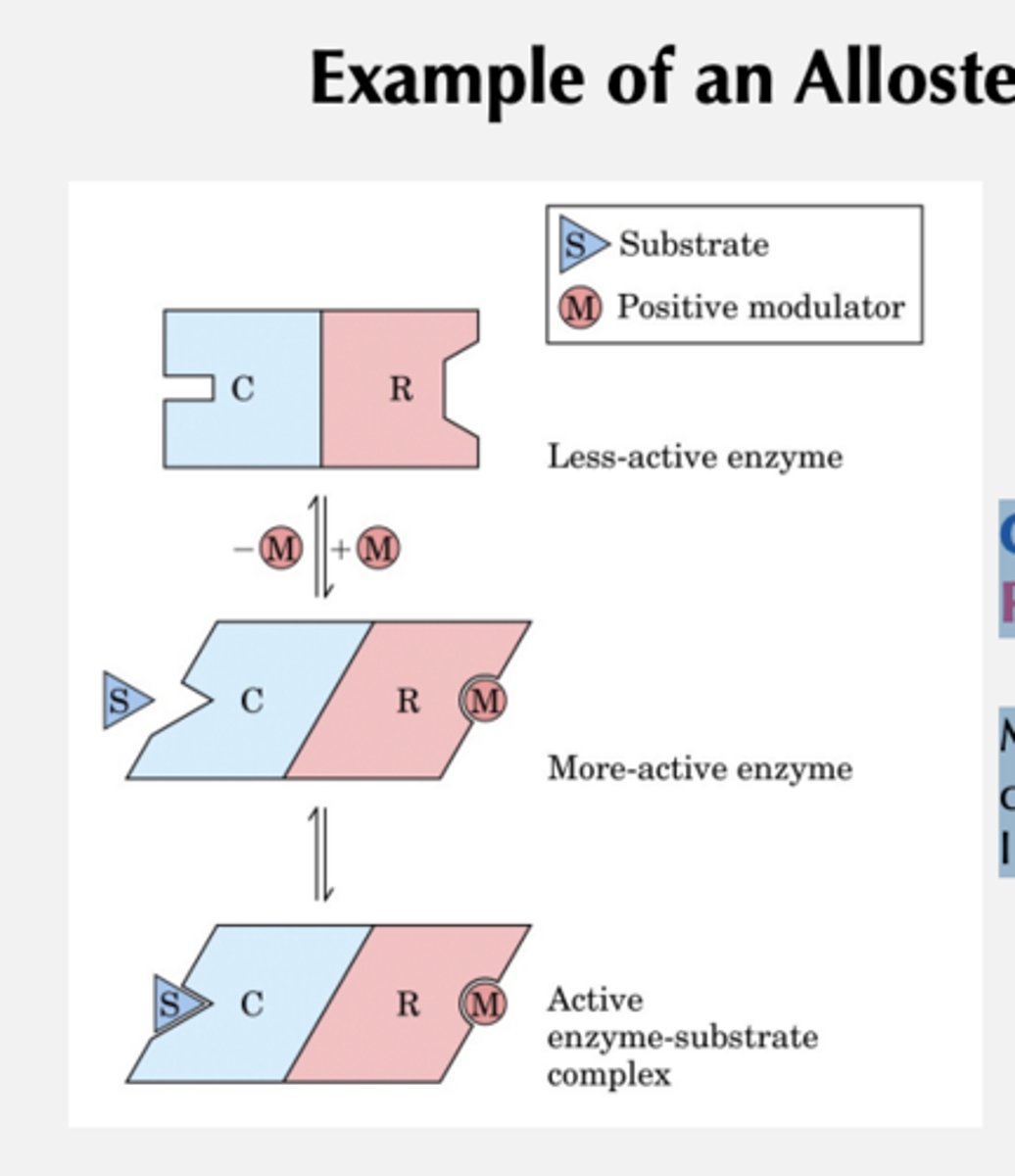
Example of an Allosteric Enzyme
C = catalytic subunit
R = regulatory subunit
Modulator binding to R induces conformational change in C -> Increase in catalytic activity
Enzyme activity can be regulated. Regulation can be:
-noncovalent modification
– covalent modification
– irreversible
– reversible
Affects mainly enzymes that catalyze reactions at break points of metabolic pathways
Regulation by Reversible Covalent Modification
Phosphorylation: Catalyzed by kinases
• Phosphorylation causes conformational changes in enzyme, which usually activate the enzyme
Dephosphorylation
Catalyzed by phosphatases
• Very important in signal transduction cascades (1/3 of eukaryotic proteins are phosphorylated!)
Phosphorylation sites
Hydroxyl group of Ser, Thr, Tyr e.g.
More Reversible Covalent Modifications
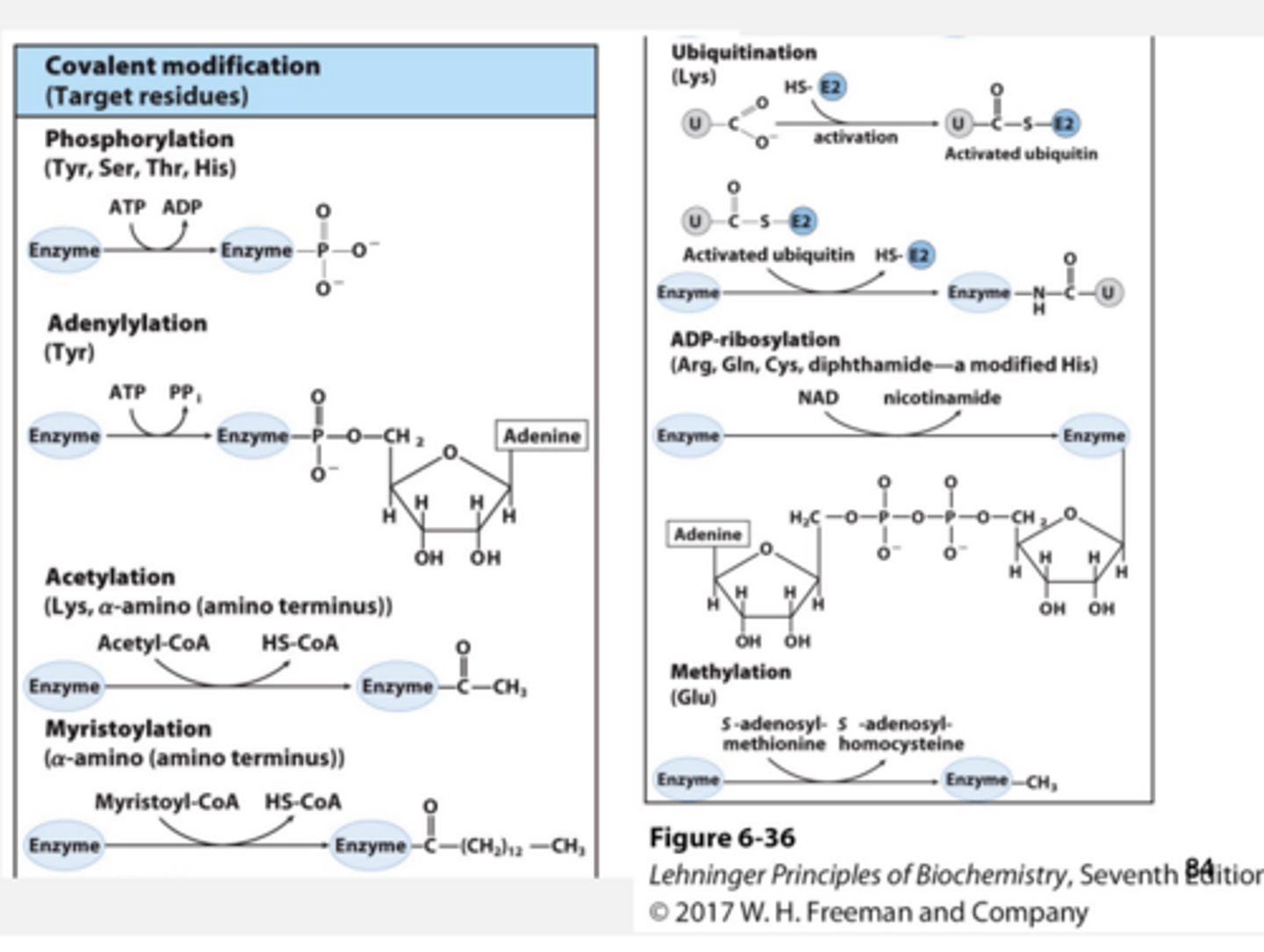
Activation by irreversible covalent modification Example:
Zymogen
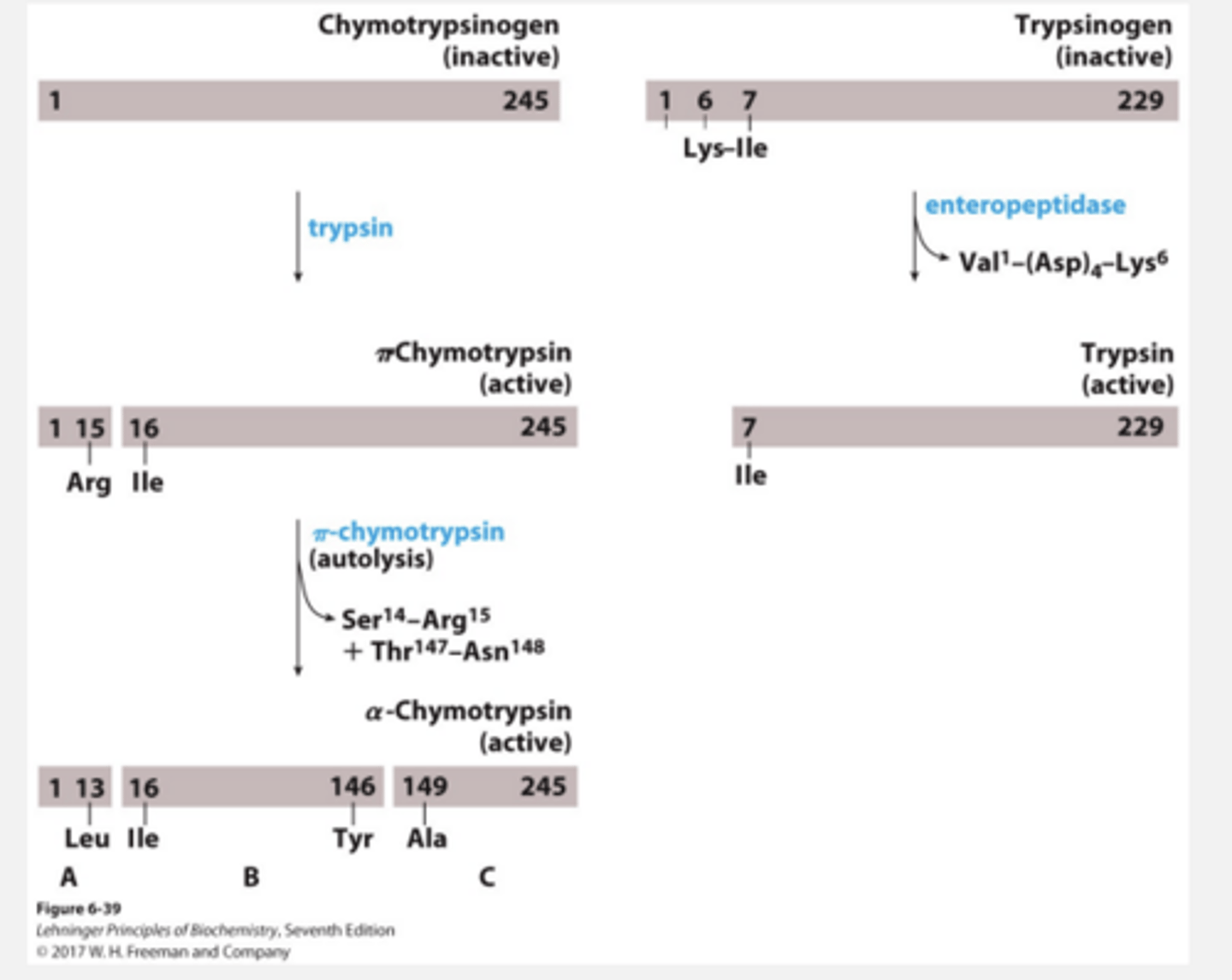
Iclicker