Bio Final Exam 1
5.0(1)
Card Sorting
1/32
Earn XP
Description and Tags
Last updated 3:10 PM on 4/27/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
1
New cards
All the organisms on your campus make up
A community
2
New cards
Systems biology is mainly an attempt to
Understand the behavior of entire biological systems by studying interactions among its component parts.
3
New cards
Which of the following best demonstrates the unity among all organisms?
the structure and function of DNA
4
New cards
A controlled experiment is one that
Tests experimental and control groups in parallel.
5
New cards
Which of the following statements best distinguishes hypotheses form theories in science?
Hypotheses usually are relatively narrow in scope; theories have broad explanatory power.
6
New cards
Which of the following is an example of qualitative data?
The fish swam in a zigzag motion.
7
New cards
Which sentence best describes the logic of scientific inquiry?
If my hypothesis is correct, I can expect certain test results.
8
New cards
In the term trace element, the adjective trace means that
The element is required in very small amounts.
9
New cards
Compared with 31P, the radioactive isotope 32P has
one more neutron
10
New cards
The reactivity of an atom arises from
The existence of unpaired electrons in the valence shell.
11
New cards
Which statement is true of all atoms that are anions?
The atom has more electrons than protons.
12
New cards
Which of the following statements correctly describes any chemical reaction that has reached equilibrium?
The rates of the forward and reverse reactions are equal.
13
New cards
We can represent atoms by listing the number of protons, neutrons, and electrons for example, 2p+, 2n^0, 2e- for helium. Which of the following represent the 18O isotope of oxygen?
8p+, 10n^0, 8e-
14
New cards
The atomic number of sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound.
H2S
15
New cards
What coefficients must be placed in the following blanks so the all atoms are accounted for in the products?
C6H12O6 → __ C2H6O + __ CO2
C6H12O6 → __ C2H6O + __ CO2
2;2
16
New cards
Which of the following is a hydrophobic material?
wax
17
New cards
We can be sure that a mole of table sugar and a mole of vitamin C are equal in their
number of molecules
18
New cards
Measurements show that the pH of a particular lake is 4.0. What is the hydrogen ion concentration of the lake?
10^-4M
19
New cards
Measurements show that the pH of a particular lake is 4.0. What is the hydroxide ion concentration of the lake?
10^-10M
20
New cards
A slice of pizza has 500 kcal. IF we could burn the pizza and use all the heat to warm a 50-L container of cold water, what would be the approximate increase in the temperature of the water? (Note: A liter of cold water weighs about 1 kg.)
10 degree C
21
New cards
Organic chemistry is currently defined as
the study of carbon compounds.
22
New cards
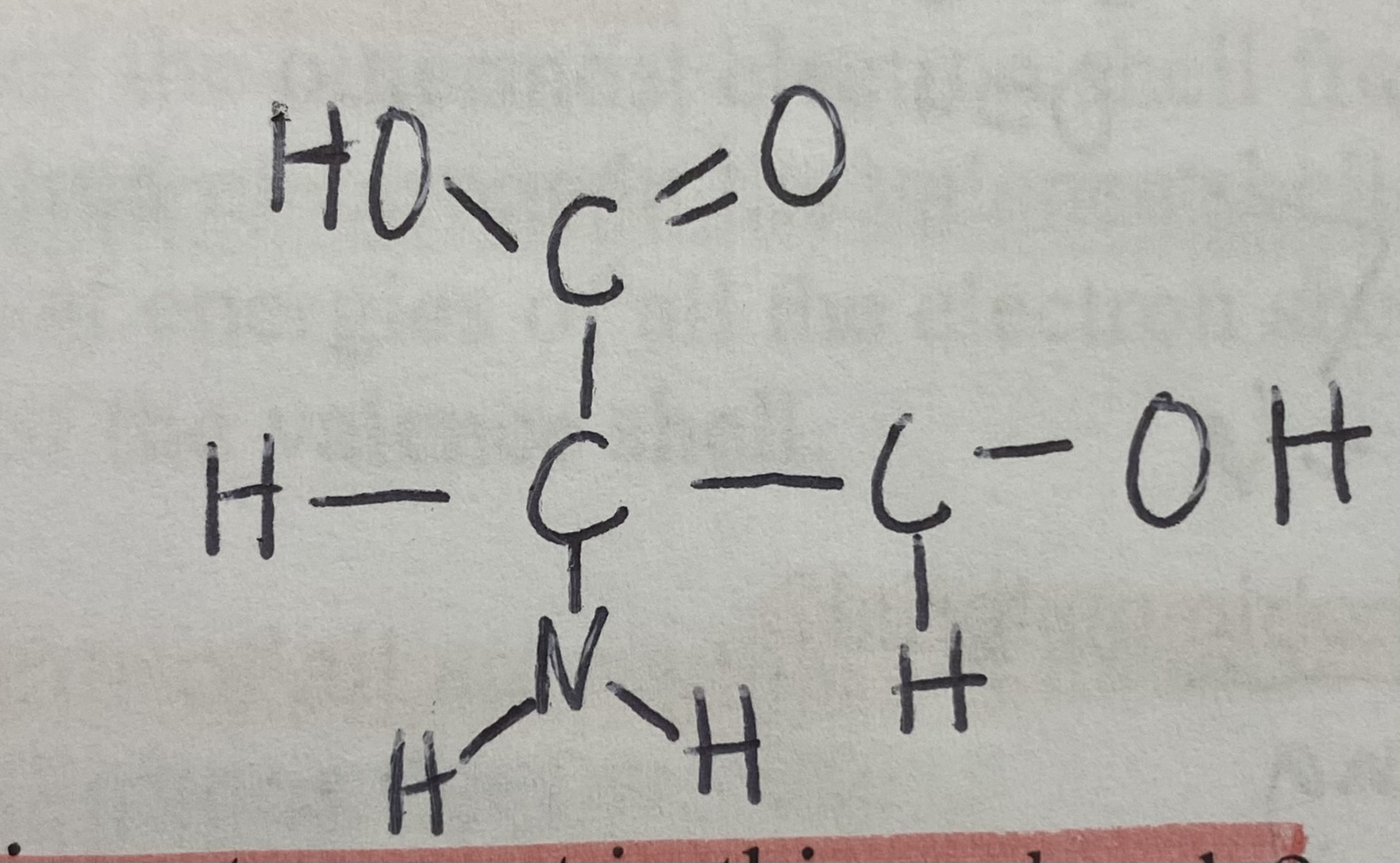
Which functional group is not present in this molecule?
Sulfhydryl
23
New cards
Which chemical group is most likely to be responsible for an organic molecule behaving as a base?
amino
24
New cards
Visualize the structural formula of each of the following hydrocarbons. Which hydrocarbon has a double bond in its carbon skeleton?
C2H4
25
New cards
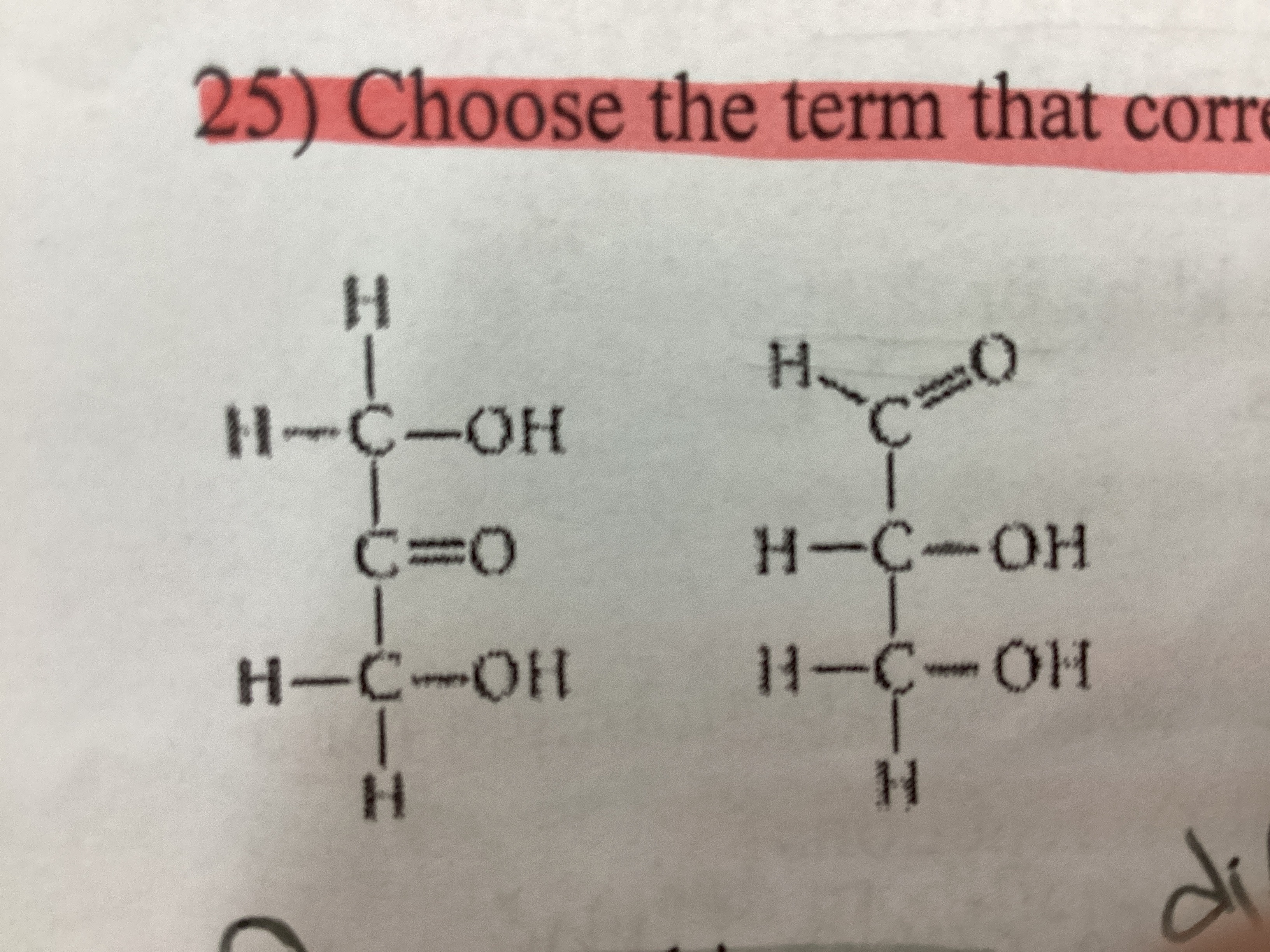
Choose the term that correctly describes the relationship between these two sugar molecules:
Structural isomers
26
New cards
Which action could produce a carbonyl group?
the replacement of the -OH of a carbonyl group with hydrogen
27
New cards
Which of the following categories includes all others in the list?
carbohydrate
28
New cards
The enzyme amylase can break glycosidic linkages between glucose monomers only if the monomers are in the form. Which of the following could amylase break down?
Glycogen, starch, and amylopectin
29
New cards
Which of the following is true of unsaturated fats?
They have double bonds in their fatty acid chains.
30
New cards
The structural level of a protein least affected by a disruption in hydrogen bonding is the
primary level
31
New cards
Enzymes that break down DNA catalyze the hydrolysis of the covalent bonds that join nucleotides together. What would happen to DNA molecules treated with these enzymes?
The phosphodiester linkages of the polynucleotide backbone would be broken.
32
New cards
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions?
C60H102O51
33
New cards
Which of the following pairs of base sequences could form a short stretch of a normal double helix of DNA?
5’-ATGC-3’ with 5’-GCAT-3’