electron config and CFSE
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
82 Terms
what does paramagnetic mean?
there are unpaired electrons
what does diamagnetic mean?
there are no unpaired electrons
what are the 5 d orbitals?
dyz, dxy, dxz, dz2, dx2-y2
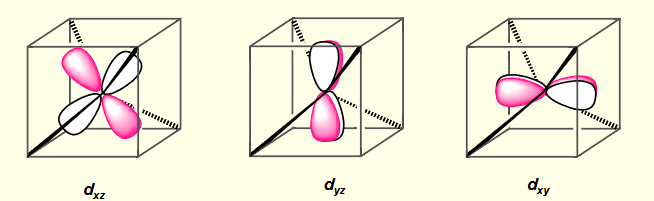
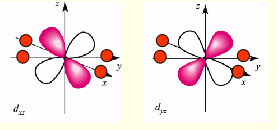
what is the shape of dyz?
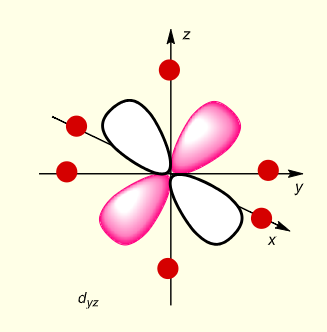
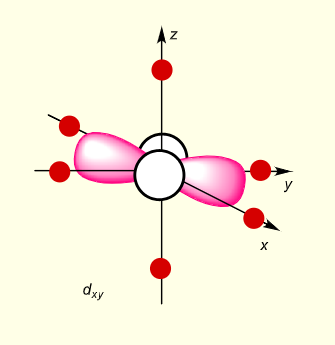
what is the shape of dxy?
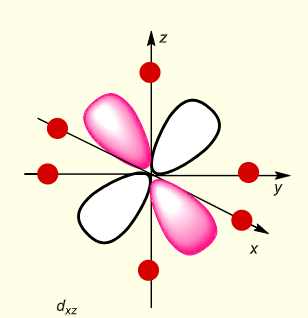
what is the shape of dxz?
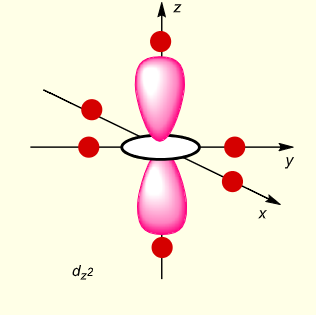
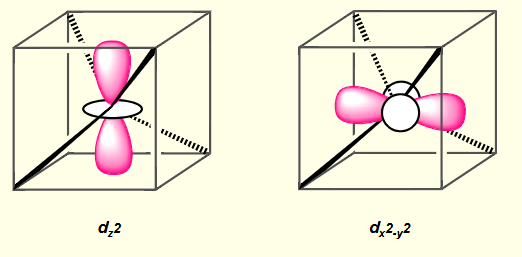
what is the shape of dz2?
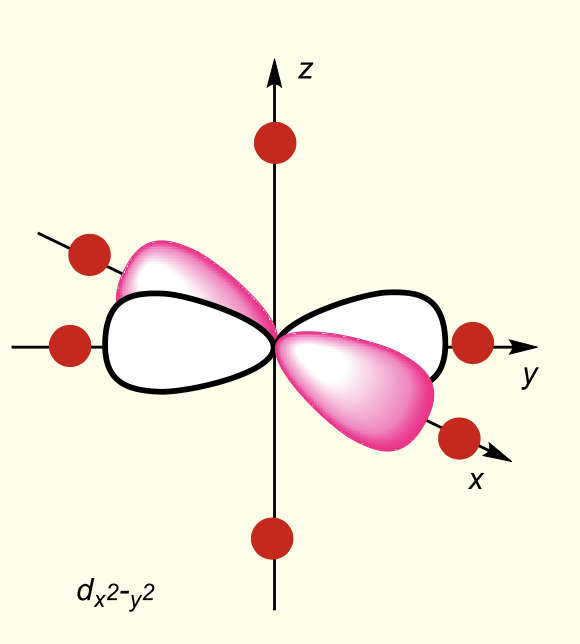
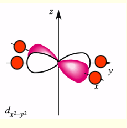
what is the shape of dx2-y2?
in an octahedral complex, which orbitals are higher energy?
dz2 and dx2-y2
the dz2 lobes are pointing directly at negative point charges on z axis = electrostatic repulsion between electron and ligands (point charges)
dx2-y2 lobes point at negative point charges on x and y axes
why are dyz, dxy, dxz lower in energy for octahedral complexes?
lobes point between negative point charges on axes
no electrostatic repulsion between electron and point charges
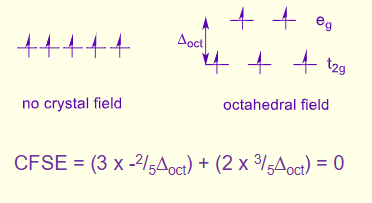
what are the two orbital energy levels for octahedral complexes?
3 lower = t2g
2 higher = eg
what is ∆oct?
what is the ratio of heights?
energy difference between t2g and eg
eg is 3/5∆oct and t2g is -2/5∆oct
draw an energy level diagram for octahedral complexes
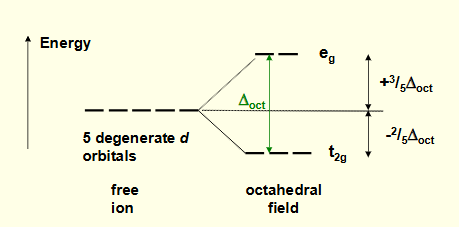
diagram of tetrahedral in cube (with cartesian axes)
axes aligned through centre of faces of cube

which are the lower orbitals for tetrahedral complexes?
dz2 and dx2-y2
lobes point between point charges (no repulsion between electron and point charge)
which are the higher orbitals for tetrahedral complexes?
dyz, dxy, dxz
lobes point near to point charges. energy destabilised due to repulsion between electron and point charge
what are the two orbital energy levels for tetrahedral?
lower = e
higher = t2
what is ∆tet?
what is the ratio of heights?
energy difference between e and t2 sets of orbitals
e is -3/5∆oct and t2 is 2/5∆oct
what is the crystal field splitting diagram for tetrahedral complexes?
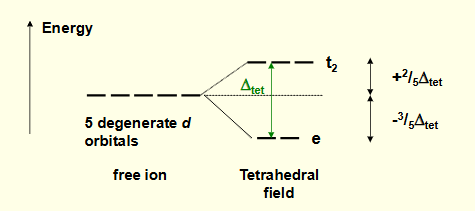
is ∆tet or ∆oct bigger? what is the relationship?
∆tet is much smaller
∆tet = 4/9∆oct for same metal and ligands
what is the crystal field splitting diagram for square planar?
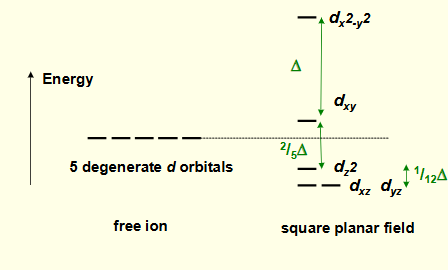
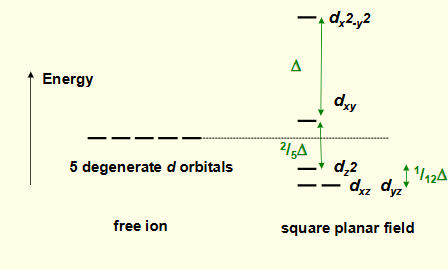
why is dx2-y2 highest in energy?
on same axes as negative point charges
most destabilised
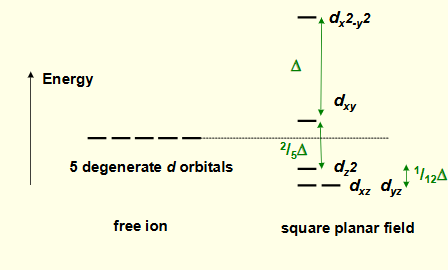
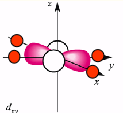
why is dxy the second highest energy?
in same plane as negative charges as negative charges
also destabilised but by a lot less
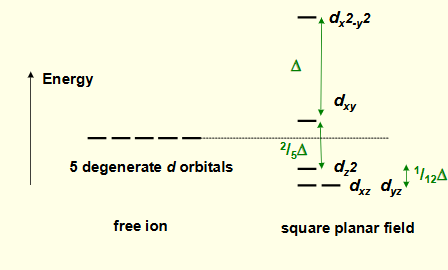
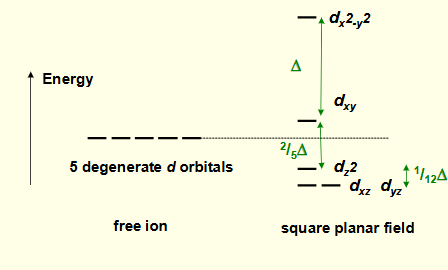
why is dz2 lower in energy?
has some orbital lobes in the same plane as negative charges
overall stabilised as most of orbital is away from negative charges
why are dxz and dyz lowest in energy?
furthest away from negative charges
stabilised and degenerate
does high or low spin maximise unpaired electrons?
high spin maximises unpaired electrons
low spin minimises unpaired electrons
how does low spin happen?
all degenerate sets of orbitals are filled before higher energy orbitals
how does high spin happen?
all d orbitals are given an electron before spin pairing
what is P?
spin pairing energy
energy cost of having two negatively charged electrons in one orbital
how to determine if a complex is low or high spin?
depends on ∆ and P (spin pairing energy)
high spin - ∆ < P
low spin ∆ > P
are tetrahedral complexes typically high or low spin?
high spin as ∆tet is small
energy cost of filling higher energy orbitals is smaller than spin pair repulsion
are square planar complexes typically high or low spin?
low spin as ∆ between dxy and dx2-y2 is large
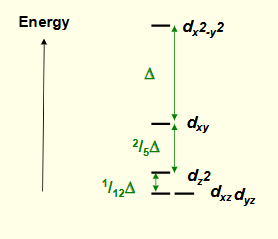
what is crystal field stabilisation energy?
energy gained from electrons filling d orbitals in crystal field
in comparison with no crystal field
how is CFSE calculated?
what is CFSE expression when there are paired electrons?
add P for the number of pairs compared to no crystal field
for which configurations is CFSE zero?
high spin d5
d10
what is the maximum stabilisation?
low spin d6
what is the general expected trend for ionic radii across the periodic table?
ionic radii to decrease
increasing effective nuclear charge, poor shielding by d orbitals
how does CFSE affect ionic radii?
is high or low spin smaller?
when there are crystal field effects the observed radii are smaller than expected
low spin ions are smaller than high spin (for same metal)
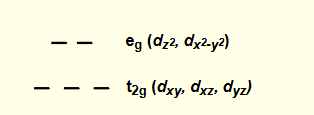
if all d orbitals contain equal number of electrons, what is distribution around metal?
spherical
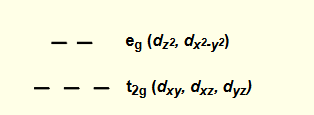
if only some d orbitals filled, what is distribution around metal?
non spherical
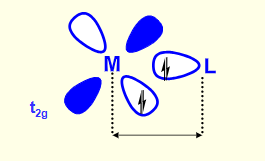
what makes M-L bond shorter?
t2g orbitals don’t point towards M-L bond but between
less repulsion between electrons in t2g and ligand
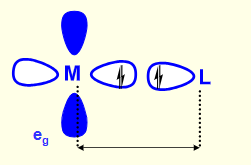
what makes M-L bond longer?
eg orbitals point towards M-L bonds - more repulsion between electrons in eg orbitals and ligand
how does radii change across periodic table from t2g0eg0 → t2g6eg0
radii decreases with an increase in effective nuclear charge and t2g occupancy
how does radii change across periodic table from t2g6eg1 → t2g6eg4
increasing eg occupancy increases overall repulsion hence radii increases
overall, how does occupancy of t2g and eg change ionic radii?
t2g decreases ionic radii
eg increases ionic radii
does low spin favour a small or large crystal field splitting parameter?
large Δ
Δ > P
does high spin favour a small or large crystal field splitting parameter?
small Δ
Δ < P
what 4 things does the size of Δ determine?
structure
magnetic properties
colour
reaction kinetics
what 5 things determine the size of Δ?
oxidation state of metal
transition series of metal
identity of metal across row
geometry of complex
identity of ligands
how does oxidation state of metal affect Δ?
greater the charge on M, the greater Δ
larger charge = ligands attracted closer to metal = stronger interaction with metal
is M(II) or M(III) complexes more likely to be high/low spin?
M(III) more likely to be low spin
Δ is larger
how does transition series of metal affect Δ (down group)?
how does P change?
Δ increases down the group
larger d orbitals have lower spin pairing energies (P)
are complexes of 2nd and 3rd more likely to be high/low spin?
2nd and 3rd row are more likely to be low spin than 1st row
Δ increases down group
how does identity of metal across row affect Δ?
across first row, for same ligand and ox state, varies irregularly across 1st row
how does geometry of complex affect Δ?
what is ratio of tetrahedral and octahedral?
square planar has large Δ
tetrahedral has small Δ
Δtet = 4/9 Δoct
how does identity of ligands affect Δ?
different ligands have different crystal field strengths
depends on position in spectrochemical series
what is the spectrochemical series?
halides are weak = low Δ
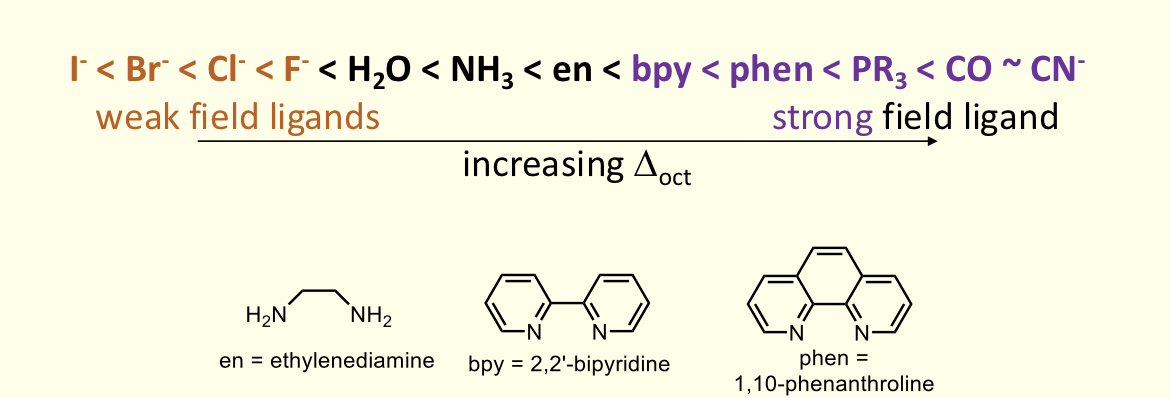
how does crystal field theory not explain spectrochemical series?
how do C≡O and halides show this?
assumes ligands are negative point charges and uses ideas of repulsion to explain d orbital splitting
halides are negatively charged and spherical so would expect to be strong
C≡O is neutral but is strong field
what are the two conditions that tetrahedral complexes tend to occur in?
small Δ
d7 electron counts
why does tetrahedral favour d7?
gives all filled or half filled sub shells (only small difference in CFSE for two geometries)
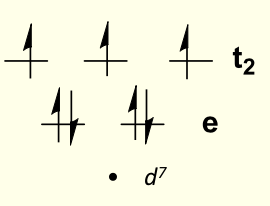
which electron configuration does square planar favour? (for small or large Δ?)
d8 when Δ is large
fully filled sub shells
large negative CFSE
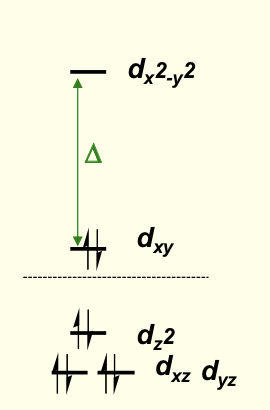
why does larger Δ affect CFSE for square planar?
pushes lower down orbitals more so more energy gained
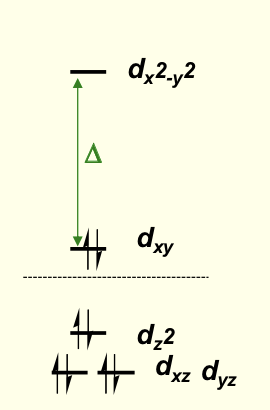
why do d10 show less of a tendency to favour particular geometries?
do not have CFSE so show less tendency to favour particular geometries
what is Jahn-Teller theorem?
any degenerate quantum mechanical state will distort in such a way to remove that degeneracy
for Cu2+ in octahedral geometry, what is electron configuration? what are the two ways to arrange this config?
how does Jahn-Teller correct this?
why is this energetically favourable?
d9
t2g6e2g3
orbitals split to break degeneracy
two electrons go lower and only one goes higher
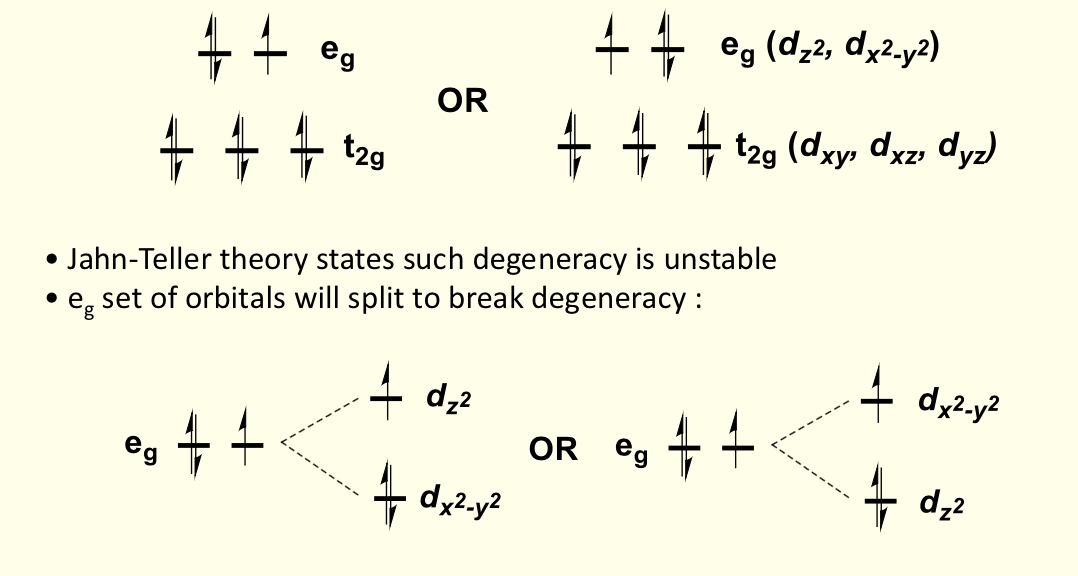
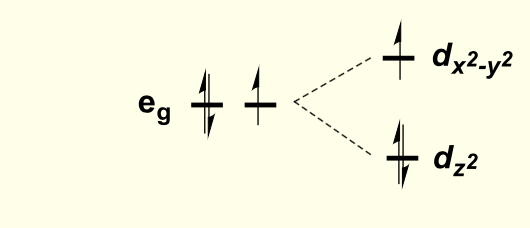
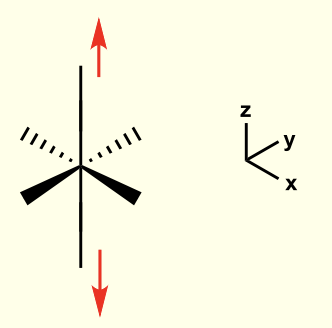
considering the directions each orbital faces, how will the overall shape of the complex stabilise orbitals to lower energy?
elongating octahedral complex along z axis
this is for when dz2 is lower
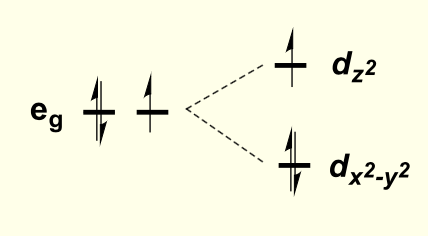
considering the direction each orbital faces, how will the overall shape of the orbital stabilise orbitals to higher energy?
compression of octahedral complex along z axis
this is for when dz2 is higher
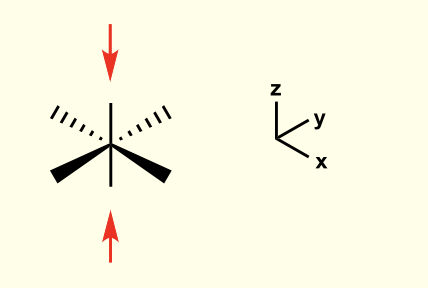
what is a tetragonal distortion?
elongation of axial bonds (z direction)
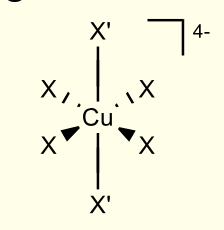
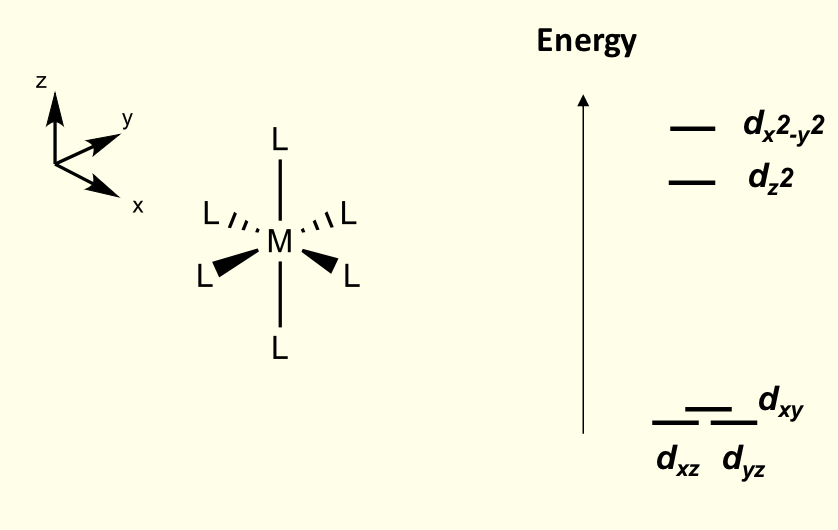
for what configurations is Jahn-Teller distortions of octahedral most pronounced for?
odd number of eg electrons
how does tetragonal geometry affect t2g orbitals?
not affected as much as don’t point directly to ligands
how does Jahn-Teller work when t2g is unevenly filled?
distortions occur so one of the d orbitals becomes lower energy
the distortions are relatively small as orbitals don’t point directly at ligands
how do individual atoms behave in paramagnetism?
behave independently unless inside external magnetic field
what is the equation for spin-only magnetic moment µso?
n is number of unpaired electrons
units are BM
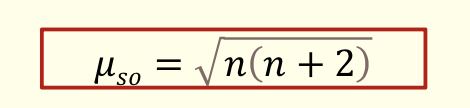
how is µeff related to µso?
approximately equal
how is µeff determined?
experimentally by measuring magnetic susceptibility of complex
measuring weight changes inside magnetic field caused by alignments of moments with the field
what colour is present if no visible light is absorbed?
white light is reflected = no colour
what causes colour in transition metal complexes?
electronic transitions of electrons in split d orbitals
what is the frequency for red and violet light?
14,300 red
23,800 violet
what is a d-d transition?
from t2g to eg
what do you need for a d-d transition?
d electrons
split d orbitals with unfilled higher energy d orbital in ground state
what are charge transfer transition?
electronic transitions from ligand to metal and vice versa