Covalent + Metallic Bonding
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
What is a covalent bond?
A shared pair of electrons
What is dative covalent bonding? / Co-ordinate bonding
When a shared pair of e- in the covalent bond comes from one of the bonding atoms
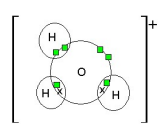
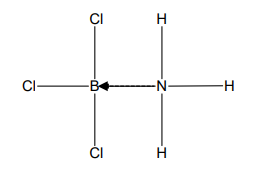
What does the direction of the arrow show?
The direction of the arrow goes from the atom that is providing the lone pair to the atom that is deficient
What is metallic bonding?
The electrostatic force of attraction between the positive metal ions and the delocalised e-
What are the 3 main factors that affect the strength of metallic bonding?
Number of protons — the more protons, the stronger the bond
Number of delocalised e- — the more delocalised e-, the stronger the bond
Size of ion — the smaller the ion, the stronger the bond