Chemistry (P1/2) - Uncertainty in Measurements
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
16 Terms
Describe mean and how it is used
Mean is an overarching representation of a group of values, obtained identically, which involves adding said values and dividing the sum by the amount of values in the group. It is used to represent data wholly for each use in calculations and elucidative purposes
Describe standard deviationand how it is used
Standard deviation refers to a measure of how spread out data values are from the mean. It is used to assess the consistency of data. Lower values indicates that the data values are close to the mean and higher values indicate the opposite.
Describe precision and how it is used
Precision refers to the proximity of data values to each other. Precision is assessed by observing the differences between individual data values and is used to assess consistency in a practical sense and serves as an indication of reproducibility.
Describe accuracy and how it is used
Accuracy refers to the proximity of data values to the true value of the quantity being measured. Accurate measurements can be obtained by repeating measurements, repeating using different instruments, using accurate/appropriate measuring instruments and using measuring instruments carefully

Describe this image
The difference between accuracy (b) and precision (a)
Describe calibration curve and how it is used
A calibration curve refers to a graph plotting the data obtained from an instrument against known values. This can be used to assess the accuracy of an instrument as an expected value can be derived using the known values
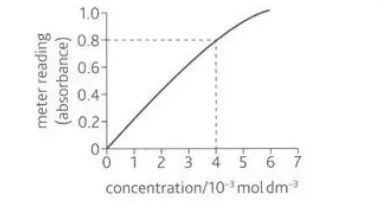
Describe this image
A calibration curve
Describe the procedure for calibrating a colorimeter

Describe standards and how they are used
They are substances with known concentrations that are used to assess the accuracy of instruments and the concentrations of other substances
Describe the procedure when adding/subtracting values that each have uncertaintities
One must add the certain values independently and then include the square root of the sum of the uncertainties
State the four criteria for determining the amount of significant figures
All non-zero digits are significant
Zeroes in the middle of a number are significant
Zeroes at the beginning of a number are insignificant
Zeroes at the end of a number and after a decimal place are significant
Describe the procedure for adding/subtracting and multiplying/dividing values with differing significant figures
The answer must not have more digits after the decimal place than either of the original values
Describe balances and differentiate between mechanical, top-loading and analytical balances
Balances are typically used to measure mass. Mechanical single pan beam balances have a capacity of 600g and a scale unit of 0.1g. Top-loading balances generally have a capacity of 300g and an uncertainty of 0.01g. Analytical electronic balances have smaller capacities, typically of 100g, and an uncertainty of up to 0.00001g.
Describe the precautions that must be taken with top-loading and analytical balances when measuring masses
Vibrations on the surface on which they are placed and air currents affect balances and can cause measurements to fluctuate and the effect they can have increases with the sensitivity of the balance and hence top-loading balances should not be placed in draughts or near air currents and analytical balances must be enclosed in a balance chamber.
In addition, objects measured on analytical balances must be room temperature as temperature differences can cause convection currents in the chamber that may disrupt the accuracy of the measurement
Describe the applications and precautions (where necessary) for measuring cylinders, volumetric flasks, volumetric pipettes and burettes
Measuring cylinders are relatively inaccurate and hence are not used in quantitative chemical analysis. Volumetric flasks are used to prepare solutions of known concentration and are designed to contain a specific volume of liquid. Volumetric pipettes are used to transfer a specific volume of solution from one container to another and are designed to account for any liquid that remains in the tip. Burette error can be minimized by using titers that excess 30cm3
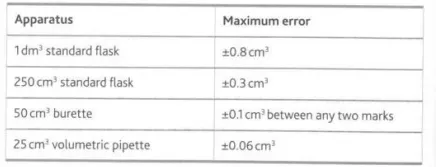
Describe this image
The uncertainties of common volumetric glassware