(CHM 1045) Lectures 1-2 Practice
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
41 Terms
Which of the following would be best characterized as a pure element?
Hf
At room temperature, the primary allotrope of sulfur is S₈. This eight-atom ring for, of sulfur is a(n) _____.
Molecular element.
A volcanic explosion that destroyed the Indonesian island of Krakatoa on August 27, 1883 released an estimated 4.3 cubic miles of debris into the atmosphere and affected global weather for years. How many cubic meters of debris were released? Save your rounding until the end, and enter your answer to the correct number of significant figures.
1.8 × 10¹⁰ m³
Most rats will die if given a dose of aspirin greater than or equal to 1.1g of aspirin per kg of body weight. If a baby aspirin tablet contains 81 mg of aspirin, how many whole tablets would a 0.75 lb rat have to consume to risk death from the dose?
5
Which of the following is an intensive physical property of a lump of coal?
color
Which of the following is an extensive physical property of matter?
length
Express the number 0.0000000018 in scientific notation.
1.8 × 10⁻⁹
Express the number 554780000040 in scientific notation to three significant figures.
5.55 × 10¹¹
The number 0.01060 has ___ significant figures.
4
A laser pulse is 2.42 × 10⁻² milliseconds. How many nanoseconds is this?
2.42 × 10⁴ ns
A student's morning commute is 21.6 kilometers. Given an average speed of 15.5 m/s, how many minutes will it take him to arrive at work?
22.8 min
An allotrope of phosphorus is P₄. This four-atom form of phosphorus is a(n) ______.
molecular element.
Sugar (sucrose, C₁₂H₂₂O₁₁) is an example of a(n)
compound
The following picture would be best described as
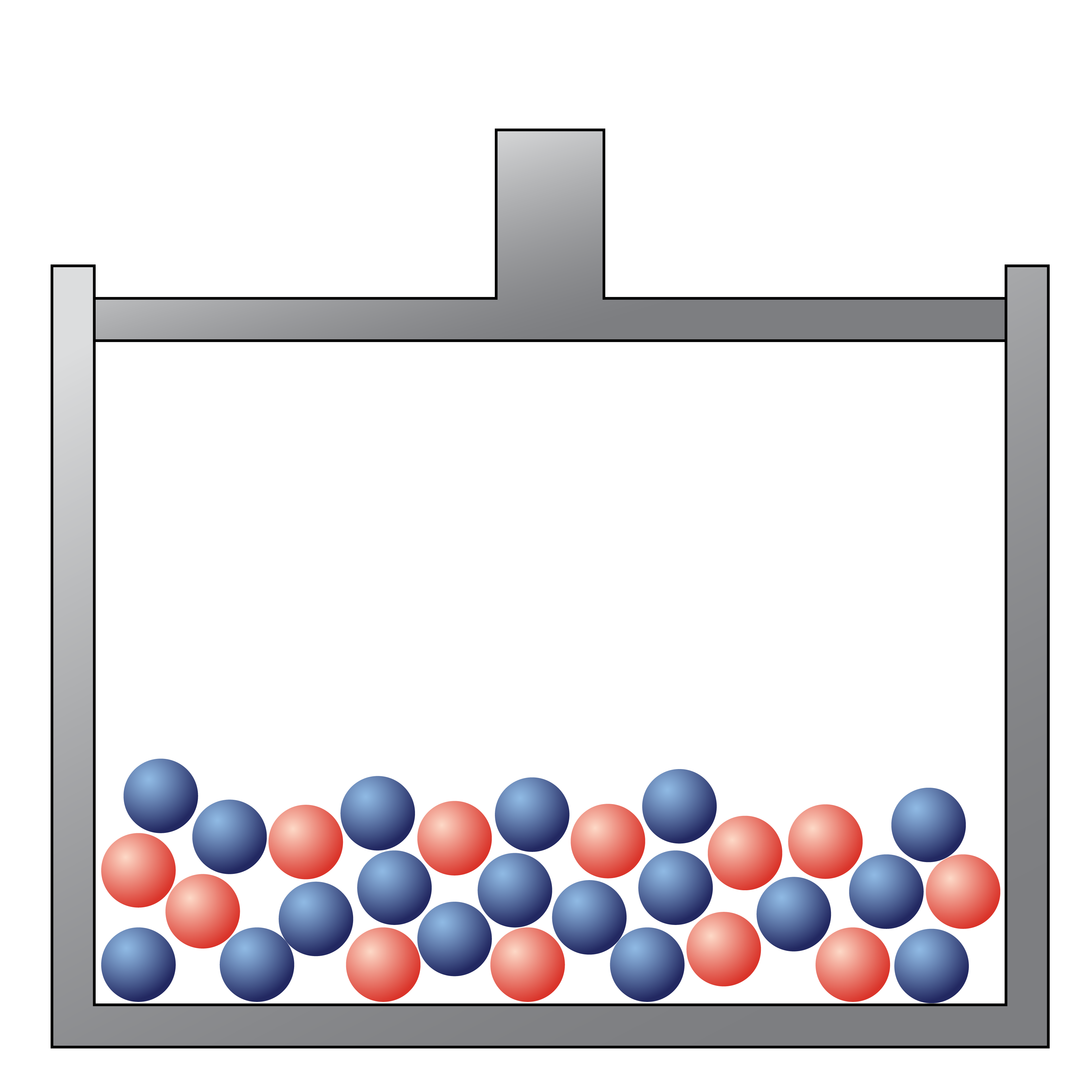
a liquid mixture
The following picture would be best described as
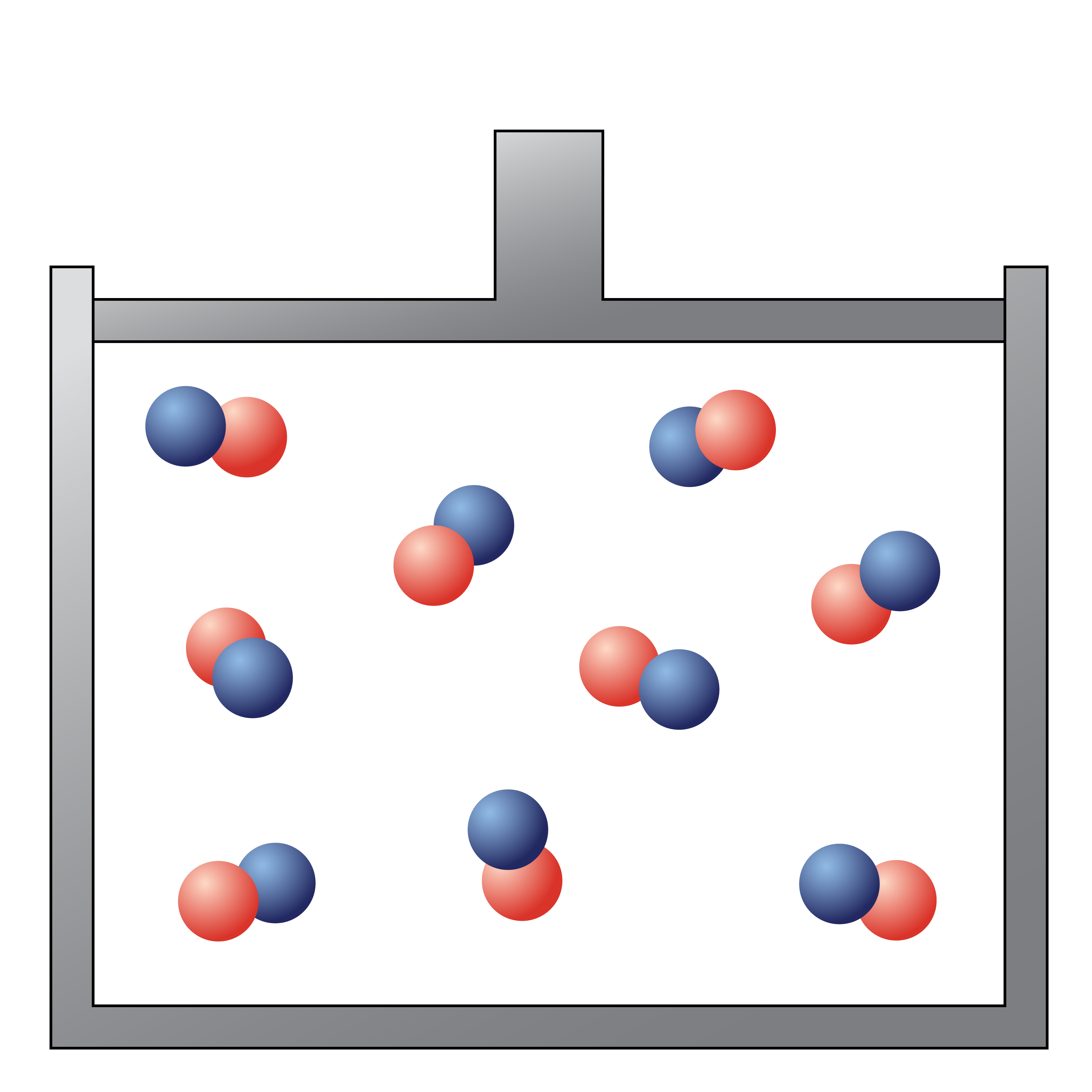
a gaseous compound
The following picture would be best described as
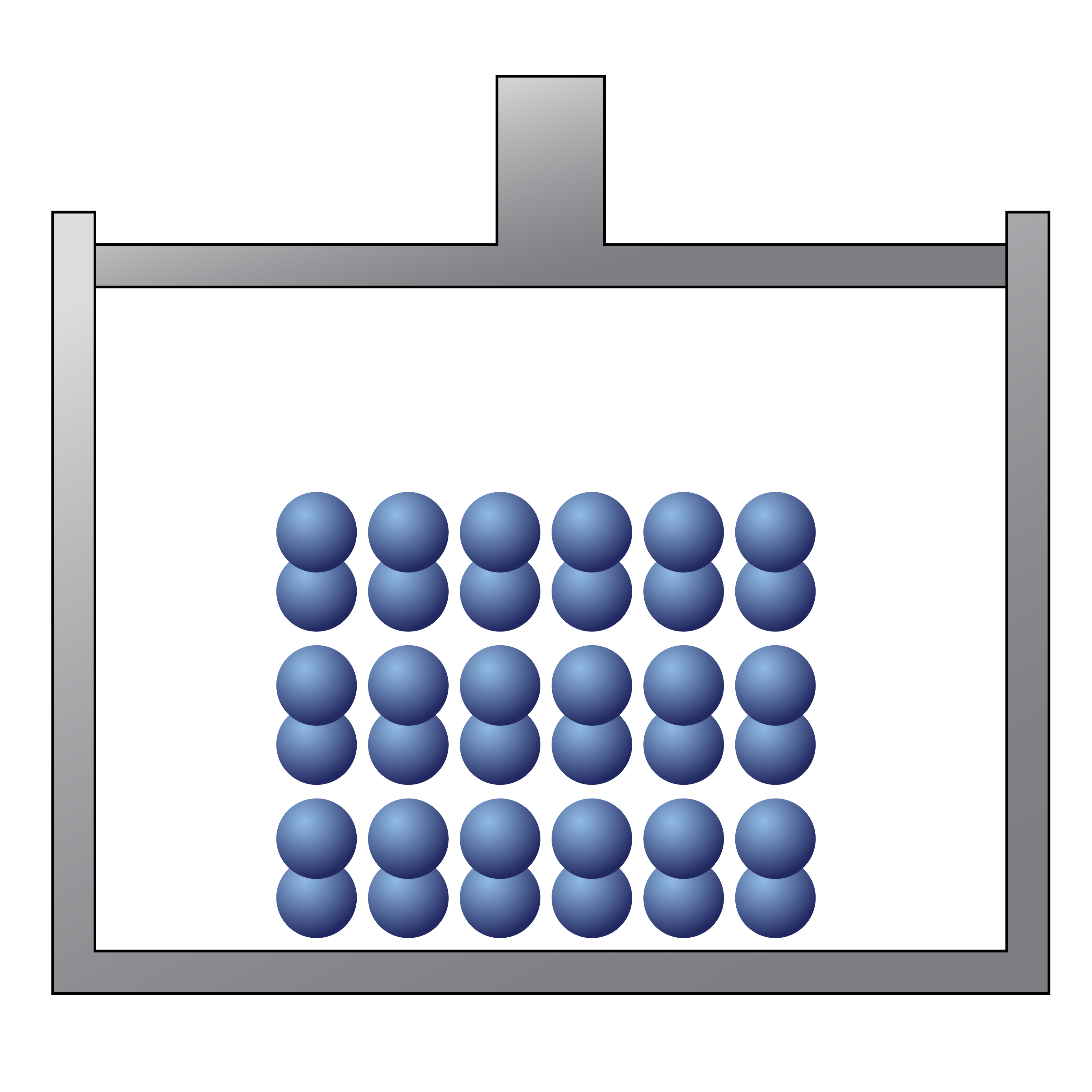
a solid element
The following picture would be best described as
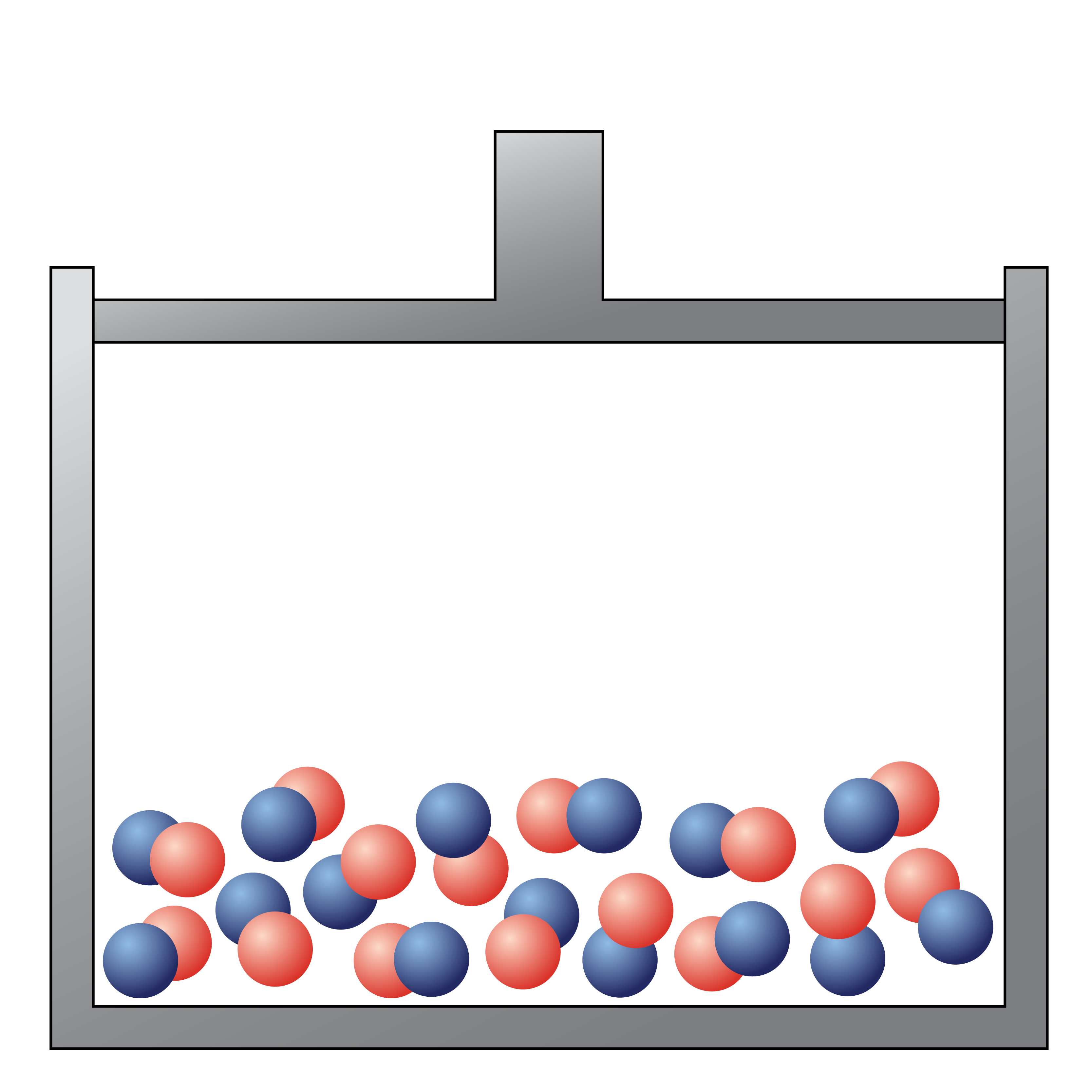
a liquid compound
Which of the following is an example of an intensive property of a bar gold?
The bar has a density of 19.2 g/mL.
Which of the following is an intensive property of one gallon of distilled water?
boiling point
Which of the following are physical properties of matter?
density
Which of the following are chemical properties of matter?
flammability
Which of the following describes a chemical change involving water?
Which of the following describes a chemical change involving water?
H₂O(l) → H₂(g) + ½O₂(g)
Convert the number shown below to decimal form.
2.6 × 10³
2600
Convert the number shown below to decimal form.
7.4 × 10⁻⁶
0.0000074
The number 0.00000620 written in scientific notation would be
6.20 × 10⁻⁶
The number 3780000 written in scientific notation would be
3.78 × 10⁶
The number 0.0509 has ___ significant figures.
3
The number 4040 has ___ significant figures.
3
The number 6.040 has ___ significant figures.
4
Convert 685 nanograms to milligrams.
6.85 × 10⁻⁴ mg
Convert 32.0 inches to meters. (2.54 cm = 1 inch)
0.813 m
Convert 1897 nanometers to centimeters.
1.897 × 10⁻⁴ cm
Cam signed up to run a 15-kilometer running race. What distance in miles will she have ran when she finishes?
9.3 miles
Which of the following is the longest length? (1 ft = 12 in, 1 in = 2.54 cm)
16.0 ft
Which of the following is the smallest mass?
5.2 mg
You are renting a car which is reported to achieve 10.5 km/L. How many miles will you be able to travel on 11.0 gallons of gasoline?
272 mi
A chemist is trying to identify an unknown metal, and finds that 25.0 cm³ of the substance has a mass of 224.43 g at 20 °C. Which of the following metals is it?
copper, d = 8.98 g/cm³
An oral suspension of the antibiotic Amoxicillin has a density of 1.72 g/cm³. What is the volume, in in³, of 0.43 lb of the oral suspension?
6.9 in³
Which will have a higher density: 1.00 g of pure gold or 1.00 kg of pure gold?
Both will have the same density.
The density of water is approximately 1 g/cm³. Which of these objects will float on water?
cedar wood block, d = 0.380 g/cm³
You have a 100 gram sample of each of the following metals. Which sample has the largest volume?
magnesium, d = 1.74 g/cm³