Physical and chemical properties of solutions I-III
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
solution
homogenous molecular dispersion of solute dissolved in solvent. The solute is typically the substance that undergoes a phase transition
characteristics of solutes in solvents
solutes interact with solvents of different extents depending on their physical and chemical properties
ideal solution
complete uniformity of intermolecular forces in a solution. Thus, solvent/solvent, drug/drug and drug/solvent interactions are equal
thermoneutral dissolution (no heat is taken or evolved in dissolution)
no shrinkage
structurally similar systems exhibit ideal behavior
surface tension, refractive index, viscosity, vapor pressure and weighted averages of each pure substance
complete uniformity
real or non-ideal solution
forces of interaction between solvent/solvent, drug/drug, solvent/drug interactions are unequal
most pharmaceutical solutions
colligative property
alteration of the properties of pure solvent due to number of solute pieces within the solution. These properties predominantly relate to the absolute number of species as opposed to the nature of the solute
effect of solute on one colligative property
lowering of vapor pressure
boiling point elevation
freezing point depression
increased osmotic pressure
vapor pressure lowering
dissolution of a non-volatile solute in a solvent will reduce the vapor pressure of a liquid as the mole fraction increases
when 2 volatile solvents are mixed the vapor pressure will be the sum of the weighted mean of each solvent
Raoult’s law
ideal substances obey Raoult’s law (states the vapor pressure of a solution is proportional to the mole fraction of solvent)
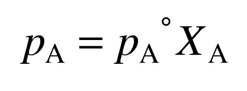
boiling point elevation
Just a solute lowers the vapour pressure (reduces evaporation), it will also elevate the boiling point of the solutions (anti-boil)
ΔTb = Elevated boiling point
Kb = Molal elevation constant
m = Molal concentration
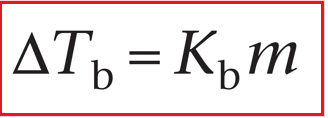
Freezing point depression
Just as a solute lowers the vapor pressure (reduces evaporation), it will also depress the freezing point of the solutions (anti-freeze)
ΔTf = Freezing point depression
Kf = Molal freezing point constant
m = Molal concentration
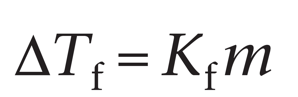
osmotic pressure
The pressure that must be applied to a solution to prevent the movement of solvent into it when solution and solvent are separated by a semi-permeable membrane (pressure due to the tendency of the solvent to move across a semi-permeable membrane from an area of low solute concentration to one of high concentration)

osmotic pressure
p = Osmotic pressure (atm)
M = Molar concentration of solution
R = Ideal gas constant
T = absolute temperature (K)

Tonicity: osmotic pressure in cells
isotonic: equal concentration of dissolved species inside the cell vs. outside the cell
Hypertonic: higher number of dissolved species outside the cell vs. inside the cell
Hypotonic: lower number of dissolved species outside the cell vs. inside the cell
dissociation influences colligative property
1m solution of a non-ionic depresses the freezing point of water by 0.512 degrees, but a ionizable substance will dissociate which influences the overall number of species in water