General Biology unit 1
1/150
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
151 Terms
What are the 5 fundamental characteristics all living organisms share
Cells
Replication
Information
Energy
Evolution
Theory
a big idea that explains why or how something happens.
It’s based on a lot of evidence and has been tested many times.
Cell theory
What are organisms made of? Where do organisms come from?
states that all organisms are made up of cells and that cells come from preexisting cells
Theory of evolution by natural selection
How are organisms related to one another?
Chromosomes theory of inheritance
How is hereditary information transmitted from one generation to the next?
Robert Hooke
devises microscope with 30x magnification
observed small compartments invisible to naked eye and named them “cells”
Anton van Leeuwenhoek
devised microscope with 300x magnification
observed single called organisms named “animalcules”
Hypothesis
Testable statement that explains something observed
Experiment
allows researchers to test effect of a single, well-defined factor on particular phenomenon
Prediction
what you think will happen in an experiment
measurable to observable results
must be correct if hypothesis is valid
Spontaneous generation
was an alternative hypothesis for the cell theory
The belief that organisms could arise spontaneously under certain conditions
Louis Pasteur’s experiment
Louis’s hypothesis: cells arise from cells and cells do not arise by spontaneous generation
Experiment:
Two glass flasks:
Both flasks had nutrient broth
One flask had swan neck open to the air
Concluded that all-cells-from-cells hypothesis was correct
Chromosomal theory of inheritance
Proposed by Sutton and Boveri
Heredity or genetic information is encoded in genes
Genes are units located on chromsomes
What was found of in the 1950s
chromosomes are molecules of deoxyribonucleic acid (DNA)
DNA is the hereditary material
Genes are segments of DNA that code for cell products
Double helix
Each strand is made up of four building blocks: A,T,C, and G
Adenine (A) pairs with Thymine (T)
Cytosine (C) pairs with Guanine (G)
D N A carries, or encodes, information needed for an organism’s growth and reproduction
James Watson and Francis Crick proposed that D N A is a double-stranded helix
The central dogma
Describes flow of information in cells
Dogma means framework for understanding
DNA codes for ribonucleic acid (RNA) which code for proteins
RNA
Molecules that carry out specialized functions in cells
Messenger RNA (mRNA) is read to make proteins
Proteins
molecules that help cells do their jobs.
they build cell structures and speed up chemical reactions that keep the cell alive.
Why is DNA copied
to pass genetic information from cell to cell or from one organism to its offspring
Copying DNA is highly accurate
What happens if a mistake is made when DNA is copied?
A change in DNA can cause changes in proteins
Proteins determine how we look and how our bodies function
DNA changes can be passed down and create differences between living things, leading to diversity
What do cells need to carry out chemical reactions?
Chemical reactions inside cells require energy
Organisms have two main nutritional needs:
Energy in the form of ATP (adenosine triphosphate)
Molecules used as building blocks for DNA, RNA, proteins, and other cell parts
How organisms get energy is a key reason life is so diverse
Plants and bacteria
Can produce sugar using energy from sunlight
Use sugar to make A T P or store it in energy-rich molecules
Can use molecules absorbed by environment as food
What is evolution?
the process by which living organisms change over generations.
What did Darwin and Wallace say about how species change?
All species are related because they come from common ancestors
Traits can change from one generation to the next
Darwin called this process descent with modification
What did Darwin learn from the finches on the Galápagos Islands? (ex of natural selection)
Different islands had finches with different beak shapes
Beak shape matched the type of food available on the island
Birds with beaks best suited for their food survived and reproduced more
Over generations, this led to descent with modification — changes in traits based on survival needs
Population
Group of individuals of same species
Living in the same area at the same time
Natural selection
explains how evolution occurs
Two conditions must be met for natural selection to occur in a population:
Individuals must vary in characteristics that are heritable—can be passed onto offspring
Within an environment, certain versions of these heritable traits help individuals reproduce more than other versions
How does natural selection work?
Traits that help organisms have more offspring become more common
Natural selection affects individuals
Evolutionary changes happen in populations over time
When populations change enough, new species can form (speciation)
Fitness
Ability of individual to produce offspring:
Individuals with high fitness produce many more surviving offspring than do others in population
Adaptation
Trait that increases fitness of individual in particular environment
Tree of life
depicts evolutionary history
family tree of organisms
describes genealogical relationships among species with single ancestral species at its base
Phylogeny: actual genealogical relationships among all organisms
How is genetic variation analyzed
Biologist study RNA and DNA from different organisms
compare sequences or building blocks (A,T,C,G)
Fewer sequence variations between two species may indicate closer relationships
What are the three major groups of organisms indicated by the tree of life
Eukaryotes (have nucleus) = Eukarya
Two groups of prokaryotes (lack nucleus) = Bacteria and Archaea
Taxonomy
Taxonomy: the effort to name and classify organisms
Taxon: a named group of organisms
Domain: highest taxonomic level, created by Woese
Three domains: Bacteria, Archaea, Eukarya
Phylum: a major lineage within a domain
What did Carolus Linnaeus contribute to classification?
Created the classification system still used today (1735)
Gave each organism a unique two-part scientific name: genus + species
Genus: group of closely related species
Species: individuals that regularly breed together or have distinct characteristics from other species
Rules for naming a species
An organism’s genus and species designation is called its scientific name or Latin name:
Scientific names are always italicized
Genus names are always Capitalized
Species names are not capitalized:
For example, Homo sapiens
Null hypothesis
the idea that nothing is happening.
It says there is no difference, no effect, or no relationship.
Food competition hypothesis
Argues that long necks evolved because those with long necks can reach food unavailable to other mammals
Simmons and Scheepers tested this
Predictions:
Neck length variable among giraffes
Neck length in giraffes heritable
Giraffes feed high in trees
The sexual competition hypothesis
giraffes evolved long necks because:
Longer-necked males win more fights than shorter-necked males
Longer-necked males can then father more offspring
Data support this hypothesis
Data refutes food competition hypothesis
Question is not closed—all hypotheses must be tested rigorously
Important characteristics of good experimental designs
must have a control group
experimental conditions must be constant
repeating test essential
use large sample size
Independent variable
what’s being tested or manipulated
Dependent variable
what is observed/measured
Chemical evolution
how life started from non-living chemicals.
Simple chemicals slowly formed more complex carbon-based molecules
These molecules eventually formed one that could copy itself
Once that happened, life began and biological evolution started
What are the four types of atoms make up 96% of matter in organisms
Hydrogen, carbon, nitrogen, and oxygen
Basic Atomic Structure
The nucleus is in the center and has:
Protons (positive charge +1)
Neutrons (no charge)
Electrons move around the nucleus:
Negative charge −1
If an atom has the same number of protons and electrons, the charges cancel out
➡ The atom is electrically neutral
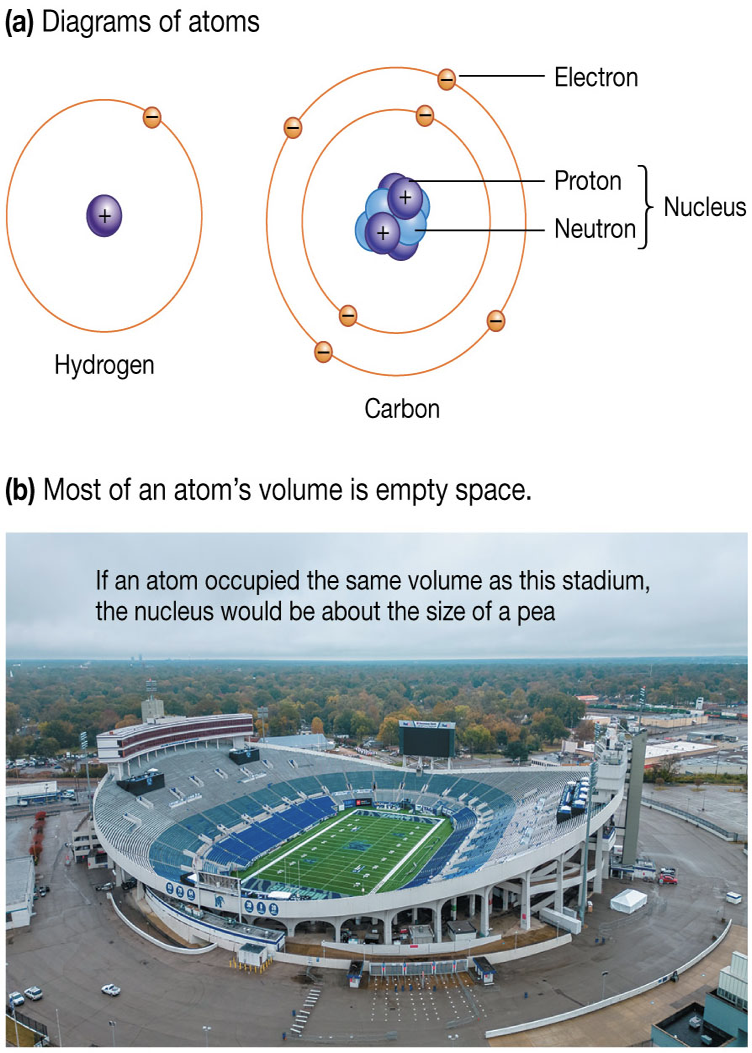
Atomic and Mass number
Elements: Consist entirely of a single type of atom
Atomic number:
Characteristic number of protons in nucleus of any atom
Written as subscript left of its symbol
Mass number:
Sum of protons and neutrons in atom
Subtract mass and atomic numbers to find neutron number
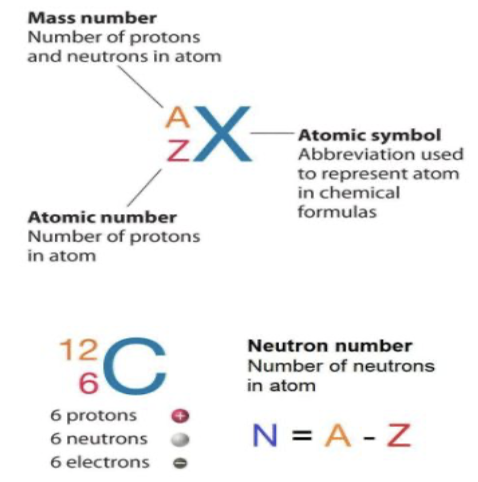
Dalton (Da)
Each proton and each neutron has a mass of one dalton (D a)
Mass of electron so small that it can be ignored
Therefore, mass of atom is equal to its mass number
what is an isotope
The number of protons never changes
The number of neutrons can change
Same element + different neutrons = isotope
Isotopes have different masses
Example: All carbon atoms have 6 protons
What is atomic weight
The average mass of all the element’s naturally occurring isotopes
Based on how common each isotope is
Example: Carbon’s atomic weight is 12.01 because carbon-12 is most common
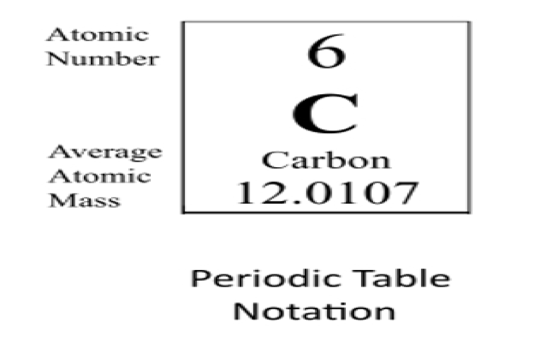
How are electrons arranged in an atom?
Electrons move in orbitals around the nucleus
Each orbital can hold 2 electrons
Orbitals are grouped into electron shells (1, 2, 3…)
Smaller numbers = closer to nucleus
This arrangement affects how elements behave
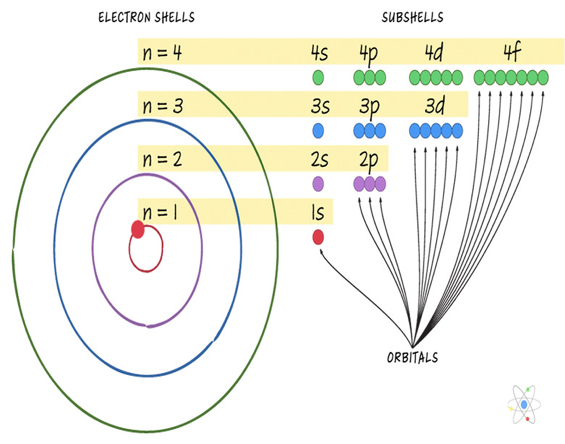
What are electron shells?
Shells hold orbitals
1 orbital → 2 electrons, 4 orbitals → 8 electrons
Electrons fill inner shells first, then outer shells
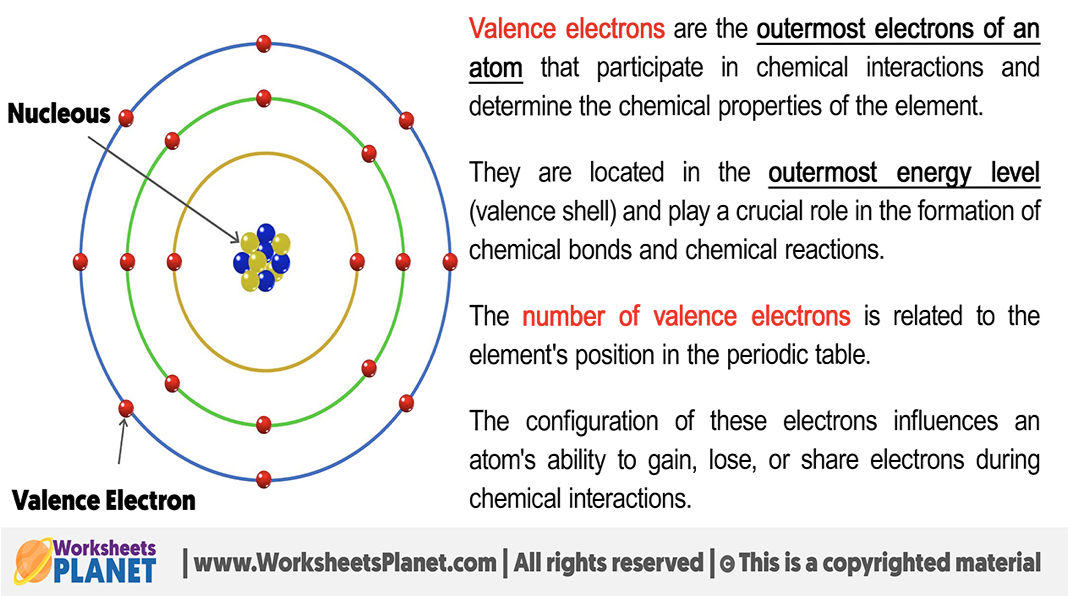
What is the valence shell and valence electrons?
Valence shell = outermost electron shell
Valence electrons = electrons in the valence shell
Valence of an atom = number of unpaired electrons
Different atoms have different numbers of unpaired electrons
How do atoms become stable?
Atoms are most stable when their valence shell is full
Shells can be filled by forming chemical bonds
Covalent bonds = sharing unpaired valence electrons between atoms
Atoms connected by bonds form a molecule
How do hydrogen atoms become stable?
Hydrogen’s valence shell isn’t full (needs 2 electrons)
Two hydrogen atoms share electrons
Sharing fills their outer shells → more stable
What are compounds and how do electrons behave in them?
Compounds = molecules made of different elements
Electrons in covalent bonds are not always shared equally
Atoms pull electrons toward themselves differently → called electronegativity
What is electronegativity?
how strongly an atom pulls electrons toward itself
Depends on:
Number of protons (more protons → stronger pull)
Distance of valence shell from nucleus (closer → stronger pull)
Trend: increases up and to the right on the periodic table
6 most abundant elements in the body: C, H, N, O, P, S
What are the differences between nonpolar covalent, polar covalent, and ionic bonds?
Nonpolar covalent bond: electrons shared evenly, bond is symmetrical
Polar covalent bond: electrons shared unevenly, bond is asymmetrical
In polar bonds, electrons spend more time near the more electronegative atom
Ionic bond: electrons transferred from one atom to another → creates charged ions that attract each other
what are ions?
Ion: atom or molecule with a charge
Cation: loses electron → positive charge
Anion: gains electron → negative charge
How do unpaired electrons determine bonds?
Each unpaired electron can join with another electron to make a covalent bond.
Number of unpaired electrons = number of bonds an atom can make
More than one unpaired electron → double or triple bonds
EX: Oxygen has 2 unpaired electrons → can form 2 bonds (like in H₂O).
How are molecules represented?
Molecular formulas: show types and numbers of atoms (e.g., H₂O, CH₄)
Structural formulas: show which atoms are bonded and if bonds are single, double, or triple
3D models: ball-and-stick or space-filling show molecule’s shape
Why is water important for life?
Cells are ~75% water
Water is an excellent solvent → can dissolve many substances
Substances react more easily when dissolved in water
What makes water unique
Small size
Bent shape
Highly polar covalent bonds
Overall polarity
Why is water a polar molecule and what are hydrogen bonds?
Water is polar:
Oxygen = partial negative (δ−)
Hydrogen = partial positive (δ+)
Water has bent shape
Hydrogen bonds = weak attractions between positive hydrogen of one water and negative oxygen of another
How does water dissolve substances
Water can stick to polar or charged molecules using hydrogen bonds
Molecules that mix well with water are called hydrophilic
This is why many polar or charged substances can dissolve in water
What are hydrophobic molecules?
Hydrophobic = “water-fearing”
Uncharged and nonpolar → do not dissolve in water
Stick together through hydrophobic interactions
Van der Waals forces help keep them stable when clustered
What happens in a chemical reaction?
Substance can be combined or broken down
Bonds are broken and new bonds form
Reactions are shown using chemical equations
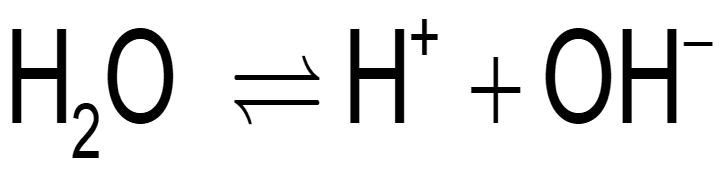
What happens when water dissociates?
Water (H₂O) can split into H⁺ (proton) and OH⁻ (hydroxide)
The reaction goes both ways:
H₂O ⇌ H⁺ + OH⁻
This is chemical equilibrium because the reaction happens both ways at the same time.
What are acids and bases?
Acids: give up protons (H⁺) → increase hydronium (H₃O⁺) in solution
Bases: take in protons (H⁺) → decrease hydronium (H₃O⁺) in solution
What is a mole
a set amount of atoms or molecules
1 mole of an element weighs its atomic weight in grams
Example: Carbon = 12 → 1 mole = 12 g
Molecular weight: add up the atomic weights in a molecule
Example: H₂O = 1 + 1 + 16 = 18 g per mole
Molarity (M)
how concentrated a solution is
measures how many moles of a substance are in 1 liter of solution
pH
pH tells if a solution is acid (low pH) or base (high pH)
Measures how many hydrogen ions (H⁺) are in the solution
Each pH number is 10 times more or less H⁺ than the next number
What do pH numbers mean and what are buffers?
Acid: pH less than 7
Base: pH greater than 7
Neutral: pH = 7 (like inside cells)
Buffers: help keep pH stable → important for homeostasis
Why is carbon important in living things?
Most molecules in organisms (except water) contain carbon
Carbon has 4 valence electrons → can form 4 bonds
Organic compounds = carbon + other elements
Can make many shapes with single and double bonds
Functional groups in organic molecules
Amino group: attracts proton → acts as base
Carboxyl group: drops proton → acts as acid
Carbonyl group: helps join molecules together
Hydroxyl group: weak acid
Phosphate group: has two negative charges
Sulfhydryl group: forms bonds between molecules
What are macromolecules and polymers?
Macromolecules: big molecules made of smaller subunits (monomers)
Polymer: many monomers linked together
Polymerization: the process of joining monomers to make a polymer
How are polymers made and broken?
Condensation (dehydration) reaction: joins monomers → loses a water molecule
Hydrolysis: breaks polymers → water is used to split the bond
What roles do carbohydrates play in cells?
Cell structure, cell identity, and energy storage.
What is a monosaccharide?
A “one-sugar” molecule; the monomer of carbohydrates.
What is an oligosaccharide?
A “few-sugars” molecule; a small carbohydrate polymer.
What is a polysaccharide?
A “many-sugars” molecule; a large carbohydrate polymer.
What are carbohydrates made of?
They are made of carbon, hydrogen, and oxygen.
The number of “sugar units” can be small (3) or very big (over 1000).
They have a carbonyl group (C=O) and lots of C-H bonds.
Not everything with C, H, and carbonyl is a carbohydrate (like formaldehyde).
Why are sugars important in cells?
Provide energy for cells
Serve as building blocks for bigger molecules
How did monosaccharides play a role in chemical evolution?
Monosaccharides like ribose were needed to make nucleotides, the building blocks of RNA and DNA
How do monosaccharides differ from each other?
Carbonyl group location:
End → aldose
Middle → ketose
Number of carbons:
3 → triose
5 → pentose
6 → hexose
Spatial arrangement of atoms:
Different arrangement of hydroxyl (–OH) groups
Shape in water:
Sugars usually form ring structures instead of straight chains
How are complex sugars (polysaccharides) made and broken?
Polysaccharides = lots of single sugars (monosaccharides) linked together
2 sugars together = disaccharide
Sugars stick together by losing water → makes a glycosidic bond
You can break them apart by adding water (hydrolysis)
What are glycosidic linkages and how do they differ?
Glycosidic linkages can form between any two hydroxyl groups of sugars
Two common types: α-1,4 glycosidic linkage, β-1,4 glycosidic linkage
Both connect the C-1 carbon of one sugar to the C-4 carbon of the next sugar
Difference: the hydroxyl on C-1 can be up or down, which changes the shape of the sugar chain
Starch
sugar storage in plants
Made of many glucose monomers
Composed of:
Forms a helix shape
Amylose = unbranched chains of starch with only 4-glycosidic linkages
Amylopectin = branched chains of starch with mostly 4-glycosidic linkages and some 6-glycosidic linkages
Branches happen about once every 30 glucose monomers
Glycogen
sugar storage in animals
Stored in liver and muscle cells
Can be broken into glucose monomers for energy
Highly branched alpha-glucose polymer, very similar to starch
Branches happen about once every 10 glucose monomers
Cellulose
structural polysaccharide
Major part of the protective layer around the plant cell wall
Made of glucose monomers joined by 4-glycosidic linkages
Every other glucose is flipped, so it:
Makes a straight (linear) molecule instead of a helix
Allows hydrogen bonds to form between parallel strands
Chitin
Structural polysaccharide
Found in cell walls of fungi and exoskeletons of insects and crustaceans
Monomer = N-acetylglucosamine (NAG)
Structure is similar to cellulose:
4-glycosidic linkages with every other monomer flipped
Forms linear strands with hydrogen bonds between them
Peptidoglycan
Structural sugar polymer in bacterial cell walls
Made of long backbones of alternating monosaccharides joined by 4-glycosidic linkages
Short amino acid chains connect the backbones with peptide bonds, giving strength
Functions of carbohydrates
Act as building blocks to make other molecules (like nucleotides and amino acids)
Provide structural materials (like fibers in cell walls)
Indicate cell identity (help cells recognize each other)
Store chemical energy for the cell
What are glycoproteins and glycolipids?
Glycoproteins: proteins with attached carbohydrates
Glycolipids: lipids with attached carbohydrates
They are involved in:
Cell–cell recognition (identify cells as “self”)
Cell–cell signaling (cell communication)
Why do carbohydrates store a lot of energy?
The electrons in carbohydrate bonds are held less tightly, giving them higher potential energy.
Tightly held electrons → low potential energy
Weakly held electrons → high potential energy
How are starch and glycogen broken down to release glucose?
They are hydrolyzed (broken down) by enzymes because they have α-glycosidic linkages.
Which enzyme breaks down glycogen, and where is it found?
Phosphorylase
Many animal cells have phosphorylase to break down glycogen into glucose.
Which enzyme breaks down starch, and what is its role?
Amylase enzymes
plays a key role in carbohydrate digestion.
Plasma membrane (cell membrane)
Separates living cells from nonliving surroundings
Acts as a selective barrier
Lets needed materials into the cell
Keeps harmful substances out
Helps life’s chemical reactions happen by holding the right chemicals in the right place
Lipids
Molecules made mostly of carbon
Do not dissolve in water
This is because they have nonpolar bonds (C–C and C–H) that don’t mix with water
Hydrocarbons
Nonpolar molecules made of only carbon and hydrogen
Hydrophobic (repel water)
Electrons are shared equally in C–H bonds, so there’s no charge