Orgo 1 Reactions
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
62 Terms
polar protic donor solvents
EtOH, MeOH, H2O, formamide (CONH2 H)
can dissolve charges
polar, aprotic, donor
DMF, DMSO, CH3CN, acetone, diethlyether
can dissolve but not stabilize - polar solvents effectively
usually SN2
Nonpolar donor protic solvent
tBuOH, acetic acid
nonpolar, donar, aprotic solvent
diethylether, THF, ethyl acetate
nonpolar, nondonar, aprotic
hexane, pentane, benzenes, ch3cl
SN2 - substitution nucleophilic bimolecular
SUBSTRATE EFFECTS
Rate = K [Nu] [substrate]
Good Lg
very weak base
Backside Attack
ALWAYS a change in stereochemistry (inversion)
SN2 - substitution nucleophilic bimolecular
NUCLEOPHILE EFFECTS
Good Nucleophile →
anions (CN-, DMF, DMSO), I- Br-, Cl-, N3-, RS-, OH-, SH2, RSH
most are negatively charged
primary or secondary alkyl halide (NO TERTIARY)
beta carbon branching is SLOWER
good nucleophile CH3>NH2>-OH>F, high electronegativity
Larger atom (more diffuses electron cloud) I > Br > Cl > F for nucleophile
so the periodic trend is Left and Down
small nucleophile
LEAVING GROUP EFFECT
good leaving group (OH<F<Cl<Br<I)
I > Br > Cl
OTs is BETTER LG THAN I-
SUPPPPPERRRR weak
OTs is o- (o=)2-s-benzene
SN2
SOLVENT EFFECT
solvent
prefers polar aprotic (F- is better than I-, C > F)
C >N >O >F
--P> S > Cl
— I
F > Cl > Br > I
in polar protic, it flips (I- is better than F-)
can hydrogen bond (SOLVATION)
form stronger ion attraction and dissolve faster (lowers energy)
STABILIZE NU, making them less reactive to substrate
less electronegative, more reactive NU
C >N >O >F
--P> S > Cl
— I
I > Br > Cl > F
larger ions are effected LESS by solvation
less polarizable by solvent
SN1 nucleophile
almost always weak!
ex: CH3OH
in SN2 it was CH3O- , the charge made it strong, but if a charge is present, usually it’ll choose SN2 > SN1. The carbocation is SO reactive it’ll take almost any nucleophile (easy)
SN1 substrate
tertiary > secondary
no primary, no carbocation
CARBOCATIONS can undergo REARRANGEMENTS if tertiary or quaternary is adjacent
SN1 solvent
MUST BE POLAR PROTIC
stabilizing the CARBOCATION
need the donating H
H2O, R-OH
SN1 LG
OTs- > I- > Br- > Cl
E2
tertiary over secondary over primary
prefer strong base
RO-, -OH
polar aprotic
strong bulky base
t-buO- (hoffman)
E1
tertiary over secondary
polar protic
weak base
H2O, CH3OH, ROH, RCOOH
Peroxide / Hbr with Alkenes
allows for faster antimarkovnikov addition
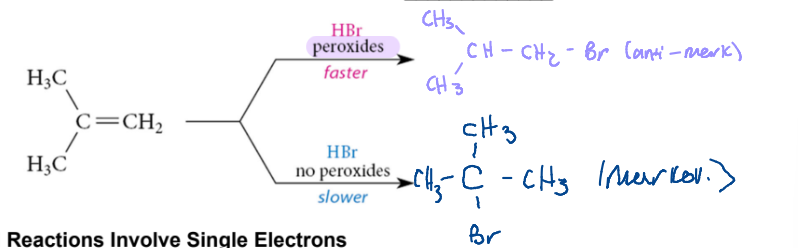
Step one of Free Radican Chain Reaction
1) Initiation
formation of the alkoxy radical
formation of the halide radical\
radical reacts with nonradical to SWAP states
* radicatls are produced from nonradicals
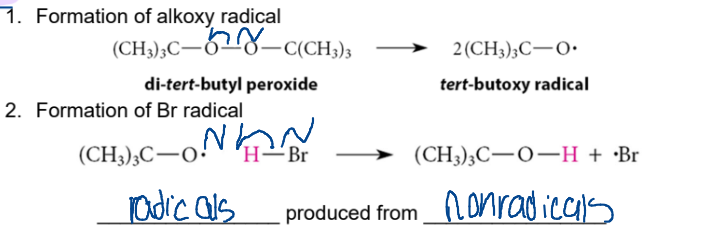
Step 2 of Free-Radical Chain Reaction
Propagation
radicals and nonradicals react to form NEW radicals and non-radicals
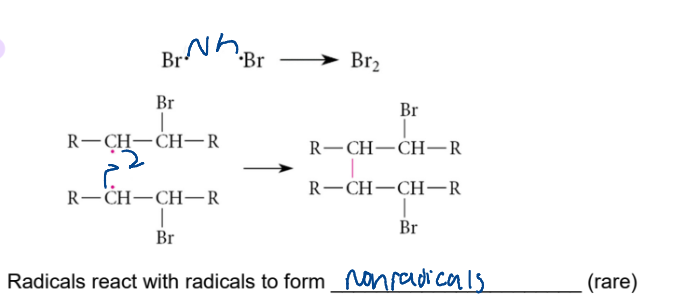
step 3 of free-radical chain reaction
termination
2 radicals react to form nonradicals
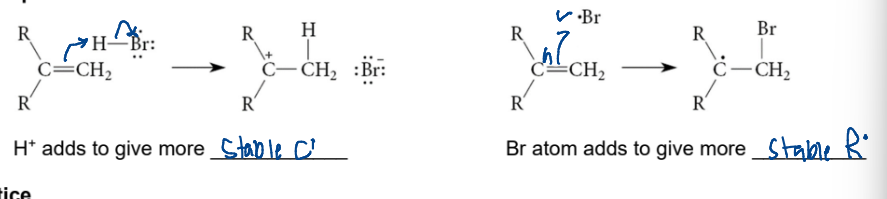
characteristics of radicals (stability)
tertiary > secondary > primary
radical goes on more substituted carbon, halide on the lesser when reacting wih alkenes (addition)
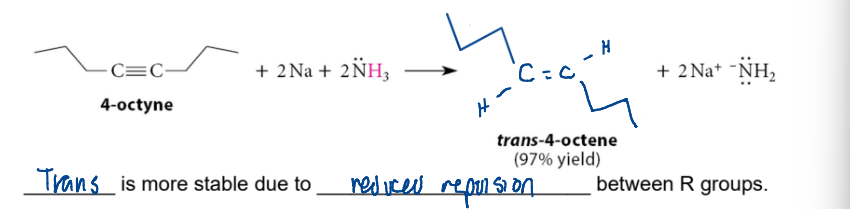
forming trans alkenes from alkynes
2Na + 2NH3
versus lindlar that does alkyne → cis alkene
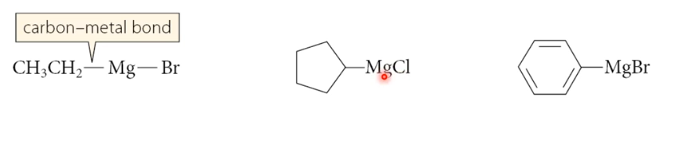
Organometallic Compounds
contain a carbon bonded to a metal (C - M)
most common:
R - Mg - X
Grignard reagents
R - Li
Organolithium reagent
formed most often from alkyl and aryl halides
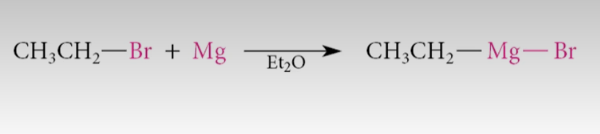
gringard reagent (Mg)
usually in nonpolar, aprotic
NEEDS TO BE APROTIC
very reactive with O2, H2O, ROH
hexane or Et2O are good
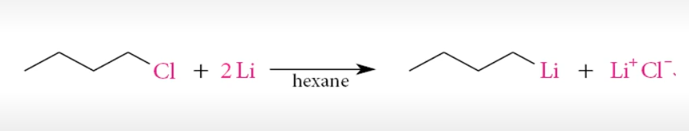
Organolithium reagent
Needs to be in an aprotic solvent
very reactive with O2, H2O, ROH
hexane or ET2O are good
Organolithium reagent acting as strong bases
the negative charge on the C allows it to react with things like H-OR, H-OH, CH3OH and TAKE the hydrogen
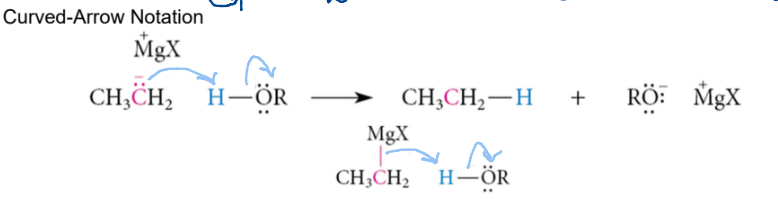
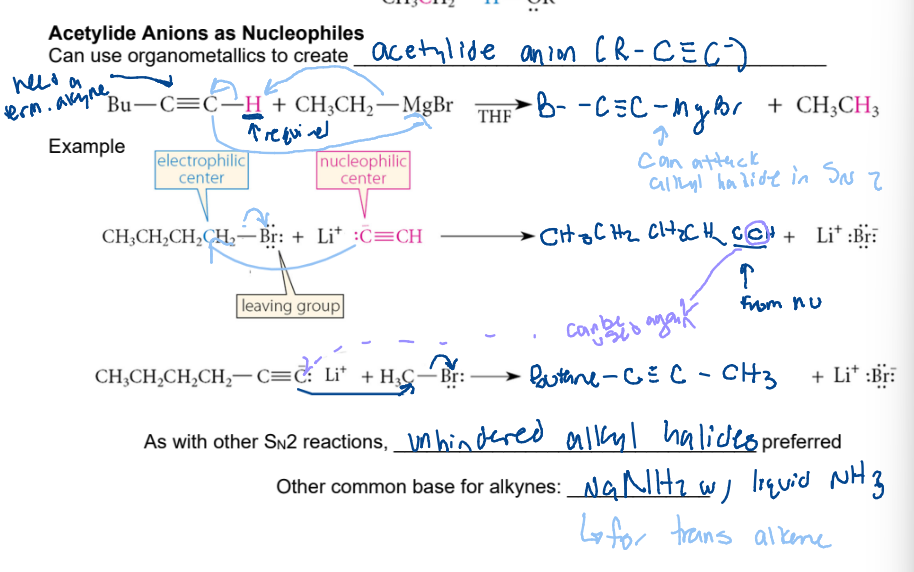
acetylide anion as nucleophiles ( R - C (tripplebond) C-)
require terminal hydrogen. anion carbon takes H. New negative charge on alkyle carbon can react with an alkyl halide and take that alkyl
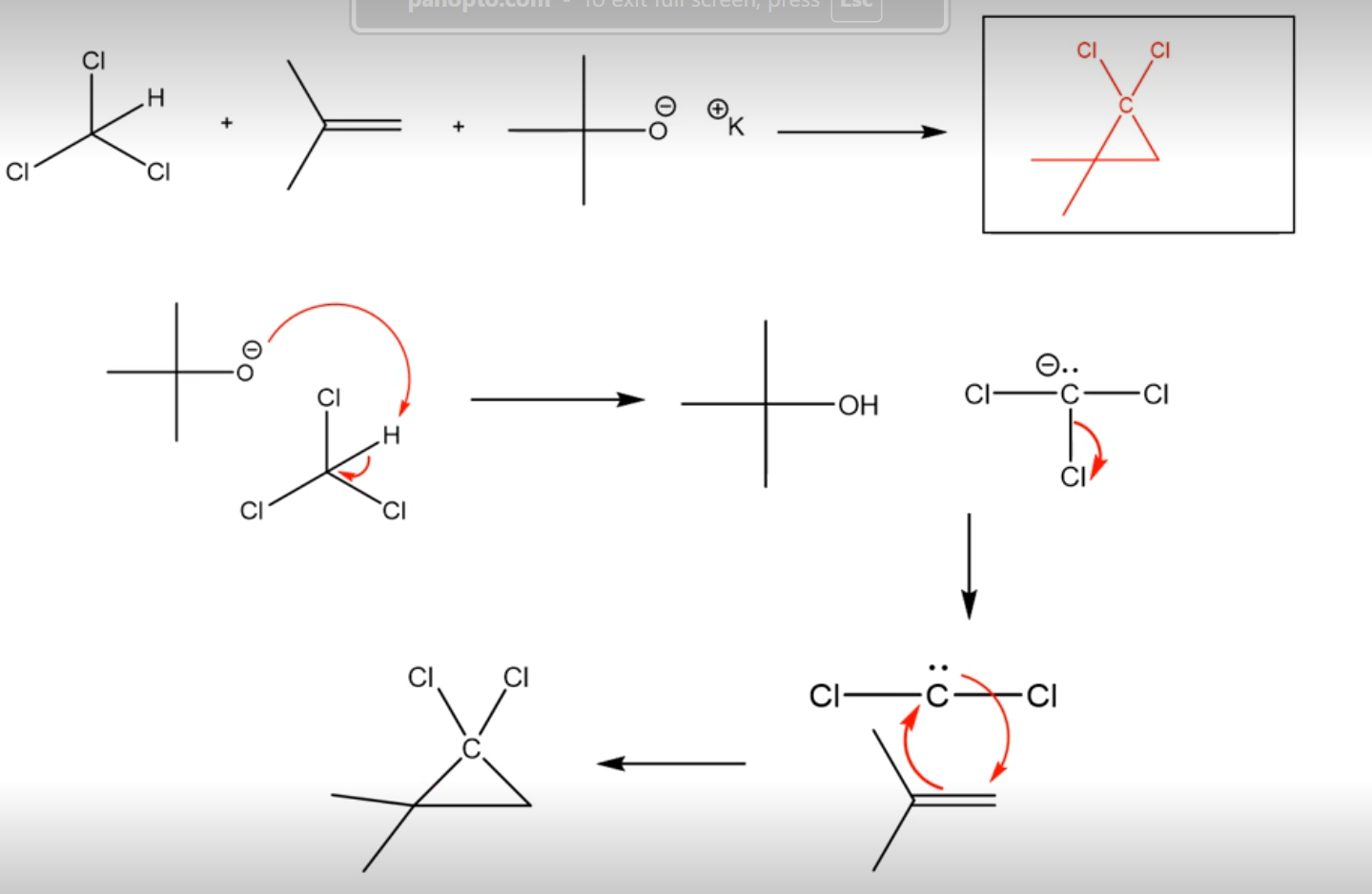
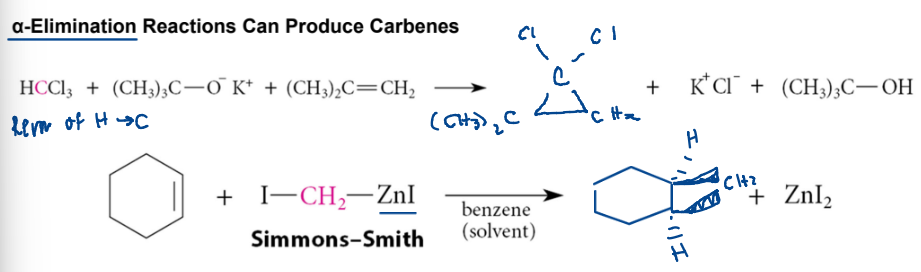
alpha elimination (producing carbenes)
strong base reacts with chloroform to make a Cl - C - Cl (not a full octet but no charge) which can act as a nucleophile and electrophile at the same time! This makes a carbene
Simmons-Smith
solvent is a benzene (nonpolar, aprotic, acceptor)
I — CH2 — ZnI
H2 Lindlar catalyst
Definition: Syn hydrogenation of an alkyne to a cis-alkene (partial reduction).
General equation:
RC≡CR' →(H₂, Lindlar)→ RC=C(R') (cis)
H₂, Pd/C catalyst
Definition: Complete hydrogenation of alkenes and alkynes to alkanes.
General equation:
RC≡CR' →(H₂, Pd/C)→ RC₂H–CR'₂H
RCH=CHR' →(H₂, Pd/C)→ RCH₂–CH₂R'
1) O₃, 2) (CH₃)₂S
Definition: Ozonolysis (reductive workup) → cleaves double bonds, giving aldehydes/ketones.
General equation:
RCH=CHR' →(1. O₃, 2. (CH₃)₂S)→ RCHO + R'CHO (or ketones depending on substitution)
HBr
Definition: Markovnikov hydrohalogenation of alkenes (no radicals).
General equation:
RCH=CHR' + HBr → RCH(Br)–CH₂R'
HBr, peroxides
Definition: Anti-Markovnikov addition of HBr via radical mechanism.
General equation:
RCH=CHR' + HBr → RCH₂–CH(Br)R'
Br₂, H₂O
Definition: Halohydrin formation (Br and OH added anti).
General equation:
RCH=CHR' + Br₂/H₂O → RCH(OH)–CH(Br)R'
NaNH₂ / liquid NH₃
Definition: Strong base that deprotonates terminal alkynes or performs double elimination to form alkynes from dihalides.
General equations:
RC≡CH + NaNH₂ → RC≡C⁻ Na⁺
RCHBr–CHBrR' →(excess NaNH₂)→ RC≡CR'
Mg, diethyl ether
Definition: Formation of Grignard reagents from alkyl or aryl halides.
General equation:
R–X + Mg → R–Mg–X
1) BH₃·THF, 2) H₂O₂, NaOH
Definition: Hydroboration–oxidation of alkenes → alcohol, anti-Markovnikov, syn.
General equation:
RCH=CHR' → RCH₂–CH(OH)R'
1) disiamylborane, 2) H₂O₂, NaOH
Definition: Hydroboration–oxidation of alkynes, giving aldehydes from terminal alkynes.
General equation:
RC≡CH → R–CH₂–CHO
Na metal, liquid NH₃
Definition: Dissolving-metal reduction of alkynes → trans-alkenes.
General equation:
RC≡CR' →(Na/NH₃)→ RC=CR' (trans)
Hg²⁺ (catalyst), H₃O⁺
Definition: Mercury-catalyzed hydration of alkynes → ketones via enol–keto tautomerization (Markovnikov).
General equation:
RC≡CH → R–CO–CH₃
CH₂I₂, Zn/Cu (Simmons–Smith)
Definition: Cyclopropanation of alkenes.
General equation:
RCH=CHR' + CH₂I₂/Zn–Cu → cyclopropane ring fused onto alkene
KOCl(CH₃)₃, DMSO (Swern oxidation)
Definition: Oxidation of alcohols → aldehydes or ketones (mild, no over-oxidation).
General equation:
RCH₂OH → R–CHO
R₂CHOH → R₂C=O
1) O₃, 2) H₂O₂ / H₂O
Definition: Oxidative ozonolysis → carboxylic acids (instead of aldehydes).
General equation:
RCH=CHR' → RCO₂H + R'CO₂H
Br₂
Definition: Halogenation of alkenes → vicinal dibromides (anti-addition).
General equation:
RCH=CHR' + Br₂ → RCH(Br)–CH(Br)R'
1) Hg(OAc)₂, H₂O; 2) NaBH₄ / NaOH
Definition: Oxymercuration–demercuration → Markovnikov hydration of alkenes without rearrangement.
General equation:
RCH=CHR' → RCH(OH)–CH₂R'
K2Cr2O7/ H2SO4 excess
turns aldehydes into carboxylic acids (21 in HW)
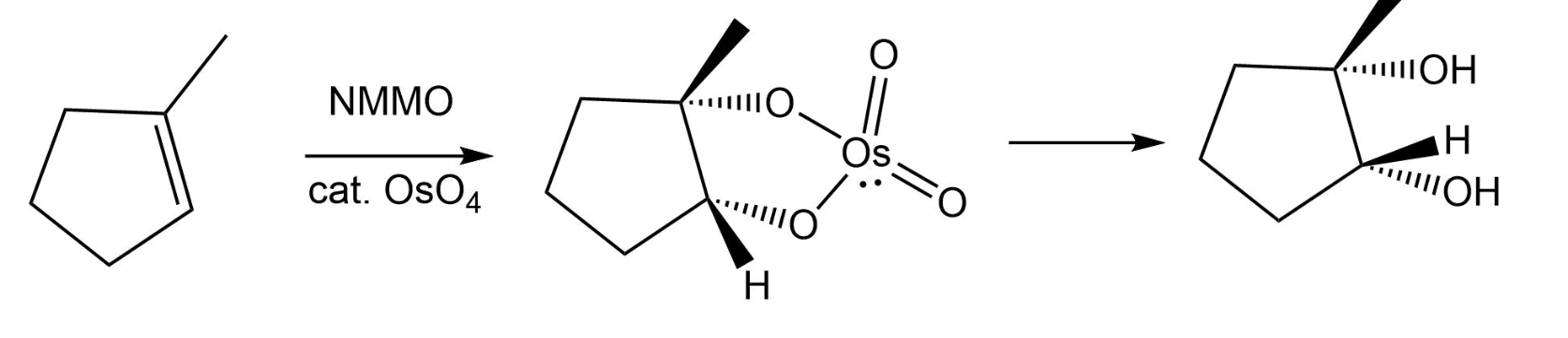
NMMO w/ cat: OsO4
alkene → alcohols added (cis to one another)
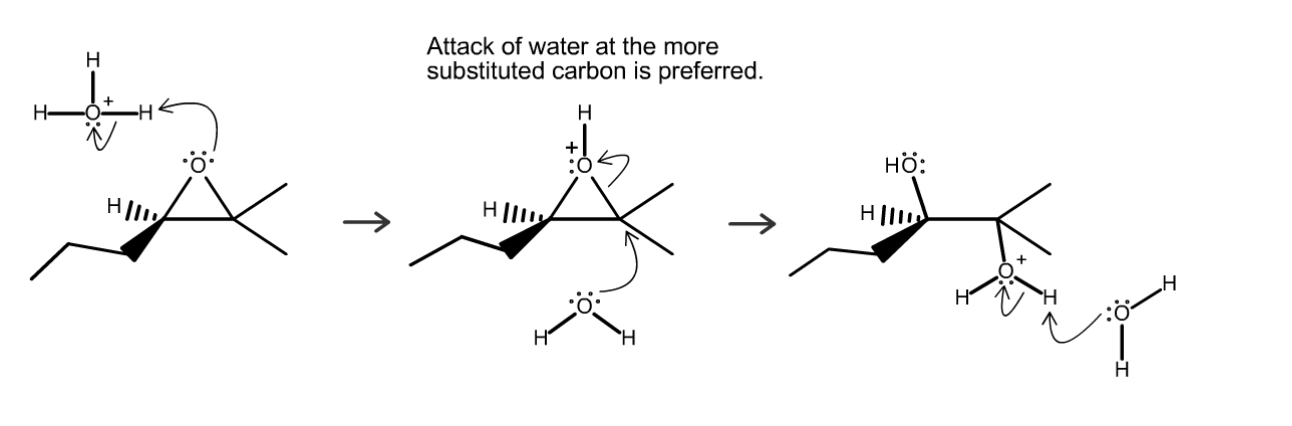
acid‑catalyzed opening of the epoxide
adds OH. Nucleophile is added to MORE substituted
mCPBA + CH2Cl2 with an alkene
forms a propane ring with oxygen (epoxide)
PCC
can make an alcohol → aldehyde
Na+ H- + HO-R (w/ K+ -OtBu solvent (polar aprotic))
formation of an alkoxide or mercaptide
Na+ -OR
Polar effect on alcohol acidity
Having an F incrreases the acidity. shorter chains are more acidic, S > O, alcohol CB are VERY acidic (h2O good lg)
H2SO4 or H3PO4 with an alkyl alcohol
dehydration and alkene formation
alcohols with HX
form alkyl bromides + water through SN1
sulfonate ester derivative of alcohols (O-S(=O)2 -O) with pyruvate in solution
removes alcohol group. sulfonate ester is a very good LG.
SOCl2 rxn with alcohols
fomation of primary halides. pyruvates are usually solvents
Ph3P-Br + Alcohol
Formation of alkyl bromide through inversion (SN2)
Phosphate LG (O-P(=O)(-OH) -OH
makes OH into a good LG and makes carbocation (E1 or SN1 rxns)
SH in the presence of HNO3 or CrO3
Oxidizes it to S(=O)2 ( -O)2
H5IO6 with alcohols
Acts similarly to ozonolysis and forms carboneles (aldehydes)
Basic‑catalyzed opening of the epoxide
Adds oh and nucleophile. Nucleophile attacks the less substituted
CrO3 H2O
Alcohol to carboxylic acid (harsh oxidizing conditions remove both h’s)