elementary steps
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
coordination (bond forming)
nucleophile creates a bond with electrophile
heterolysis (bond breaking)
molecular reverse of bond forming, a leaving group leaves (dissociates)
Sn2
Sn1
E2
E1
nucleophilic addition
a nucleophile adds to a polar pi bond (usually a carbonyl)
nucleophilic elimination
the reverse where a leaving group leaves in place of the creation of a new pi bond
electrophilic addition
an electrophilic addition, an electrophile add to non-polar alkenes or polar pi bonds
electrophilic elimination
is the reverse to electrophile addition where a substituent or H that is alpha toa carbocation is eliminated to form a pi bond
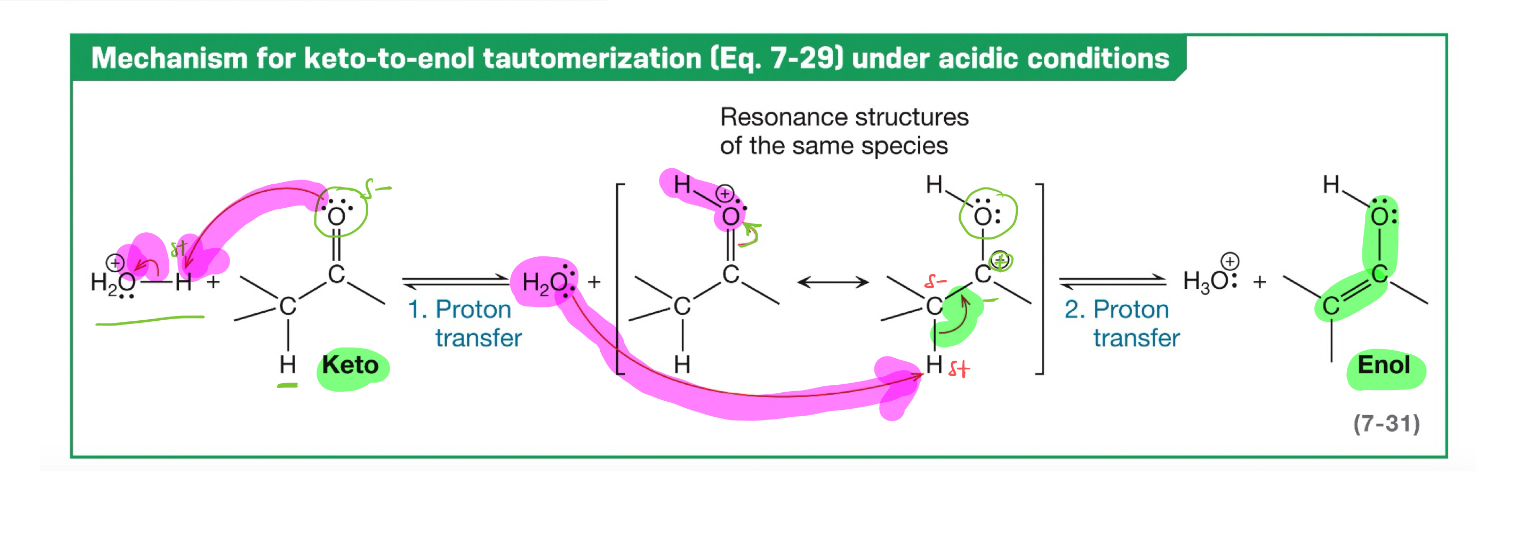
tautomerization
when two or more structural isomers (tautomers) interconvert through the rapid relocation of hydrogen atom and migration of the double bond
1, 4 conjugate
is a special type of nucleophile addition to, alpha-beta unsaturated carbonyls