Fluids and Electrolytes
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
Importance of fluid + electrolytes
maintenance of homeostasis
constantly move between intracellular and extracellular compartments
Causes of changes in movement of fluids
disease processes cause the changes in movements of fluids
Compartment Syndrome
Fluid Compartments
Intracellular compartments
Extracellular compartments (ECF further divided into interstitial fluid (around cells = 80%) and intravascular fluid (in blood vessels = around 20%)
Volumes of
Composition of body fluids
60% water
Role of water in body fluids
lubricant
transport to cells
removes waste products from cells
regulates temperature
optimum medium for cell function and chemical reactions
breaks down food particles in the GI tract
contains dissolved substances (electrolytes, gases, nutrients, enzymes, hormones)
Fluid intake
drinking (60%) - 1400-1800ml
food (30%) - 700-1000ml
oxidation (10%) - 300-400ml
Total balance: 2400-3200ml
Fluid output
urine (60%) - 1400-1800ml
faeces (2%) - 100ml
expiration of lungs (28%) - 600-800ml
skin (10%) - 300-600ml
Total balance: 2400-3200 ml
Osmosis
water moves from area of high volume to low volume through a selectively permeable membrane. (movement dependent on concentrations)
membrane impermeable to certain solutes
Diffusion
solutes move from high conc to low conc
small molecules: via concentration gradient
large molecules: via facilitated diffusion
Active transport
solutes moved across membrane AGAINST concentration gradient, process requires energy (e.g. Sodium Potassium pump)
Measuring concentration
Osmolarity vs Osmolality
Osmolality: weight based
Osmolarity: volume based
Osmolality
amount of solutes per kg of solvent (water) = weight based
precise
unaffected by temperature
Osmolarity
amount of solutes per liter of solvent (water) = volume based
calculated
measured in a lab
affected by temperature
Tonicity
Isotonic: same osmolality as body fluids (e.g. 0.9% sodium chloride)
Hypotonic: lower conc of solutes (e.g. 0.45% Sodium Chloride)
Hypertonic: higher conc of solutes (e.g. 5% Dextrose)
Electrolytes
Chemical compounds which dissociate in water to form charge particles called ions (either + or - charged)
Cations
Positively charged
Potassium (intracellular) -
Sodium (extracellular)
Calcium (intracellular)
Magnesium (intracellular)
Anions
Negatively charged
Chloride (extracellular)
Phosphate (intracellular)
Bicarbonate (extracellular)
Sodium (Na+)
generation of action potential
important in fluid/ electrolyte balance
Potassium (K+)
establish resting membrane potential
regulates pH balance
Maintains intracellular fluid volume
Calcium (Ca++)
important clotting factor
neurotransmitter release in neurons
maintains muscle tone
excitability of nervous and muscle tissue
Magnesium (Mg++)
maintains normal nerve and muscle function
maintains regular heart rate
regulates blood glucose and blood pressure
essential for protein synthesis
Chloride (Cl-)
maintains balance of anions in different fluid compartments
Bicarbonate (HCO3-)
main buffer of hydrogen ions in plasma
maintains balance of cations and anions in ICF and ECF
Phosphate (HPO4-)
essential for digestion of proteins, carbs, fats and absorption of Ca++
essential for bone formation
Sulphate (
Hormonal regulation of electrolytes
ADH by hypothalamus by osmoreceptors
Aldosterone by cortex of adrenal glands - facilitates water and sodium retention)
ADH
fluid intake regulated by thirst
fluid is lost = serum osmolarity increases
osmoreceptors in hypothalamus sense the increase = release ADH = trigger kidneys to retain water = produces thirst sensation
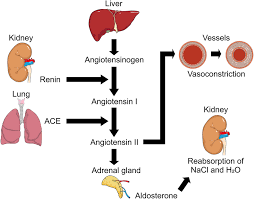
RAAS
Renin- Angiotensin – Aldosterone system
Trigger = LOW BLOOD PRESSURE
Juxtaglomerular apparatus in the kidney senses low flow and produces renin
Renin stimulates the liver to produce angiotensinogen which converts to angiotensin 1 .
Angiotensin 1 is converted to angiotensin 2 in the lungs
Angiotensin 2 triggers the adrenal glands to produce aldosterone and causes vasoconstriction
Aldosterone causes Na+ retention, K+ excretion and water reabsorption
BLOOD PRESSURE INCREASES
Causes of electrolyte imbalance
environmental conditions
disease
Fluid imbalances
Hypovolaemia (dehydration) = too little
Hypervolaemia = too much
Oedema = abnormal distribution (In the wrong places)
Hypovolaemia
Loss of blood volume and extracellular fluid
loss of water caused by vomiting/ sweating/ climate/ hormonal dysfunction (lack of ADH)
loss of blood (internal or external haemorrhage)
Hypervolaemia
Overload of fluid in blood vessels and extracellular fluid
too much water (over infusion of crystalloid fluid or polydipsia (excessive thirst))
too much intravascular fluid (over infusion of colloid fluid (e.g. blood products)
Abnormal distribution of fluids
fluid in the wrong places: cardiac failure (pulmonary/ peripheral odema), obesity (increased fluid pressure and Na+ retention), liver failure (hypoabluminaemia causing ascites), inflammation (effusions)
electrolytes in the wrong places (hyponatraemia (SIADH = damage to the hypothalamus/ side effect of some drugs)
Oedema
abnormal accumulation of fluid (mainly water) in the interstitial space
may be local (around an injury)
generally temporary and resolves without intervention, but can also be generalised (e.g. cardiac failure leads to swollen ankles because weak heart cannot pump blood up from vessels so fluid leaks into surrounding tissues)
Result of too much ADH
SIADH = body retains too much water
Result of too little ADH
body does not retain enough water
Dehydration
A consequence of insufficient intake or exessive losses, including insensible losses
Consequences of dehydration
constipation
UTI
Increased blood viscosity = increases risk of Coronary Heart Disease
Poor blood glucose control in diabetics
falls in the elderly
acute kidney injury
Hyponatraemia
a serum sodium level >145 mmol/L
a deficit of total body water relative to sodium, causing cellular dehydration
can cause headache, confusion, dizziness, brain swelling and death
Hypernatraemia
serum sodium concentration below 135 mmol/L
an excess water relative to sodium, leading to cell swelling
thirst, weakness, neuromuscular excitability, hyperreflexia, confusion, seizures, coma.
brain shrinkage caused by osmotic imbalances
Electrolyte disturbances
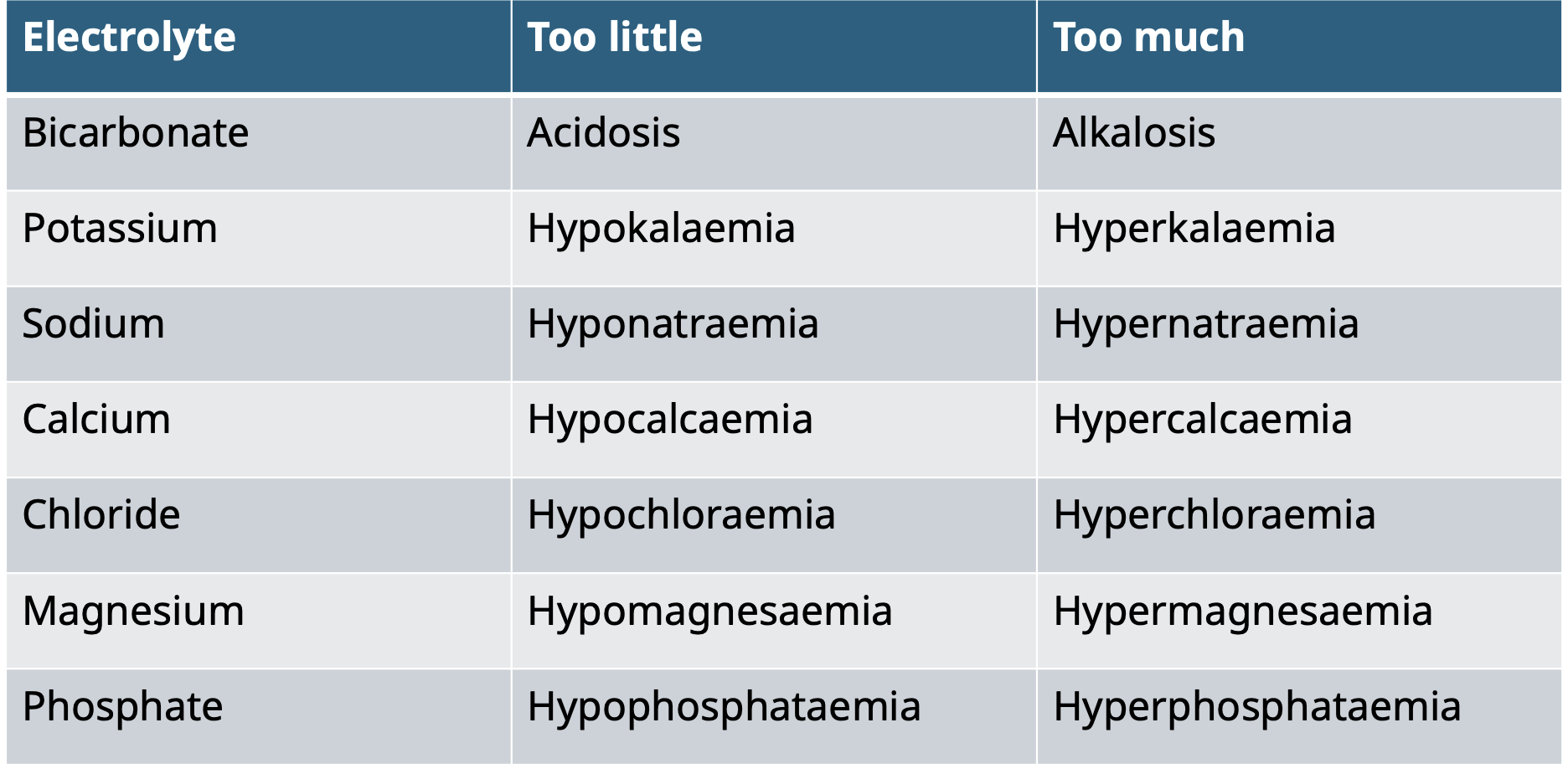
Treating electrolyte imbalances
easier to treat deficiencies than excess (like adding salt to cooking) by using supplements
Managing excess water
acetazolomide
loop diuretics (bumetanide, furosemide, torsemide)
Thiazides
Spironolactone (aldosterone antagonist), amiloride, triamterine
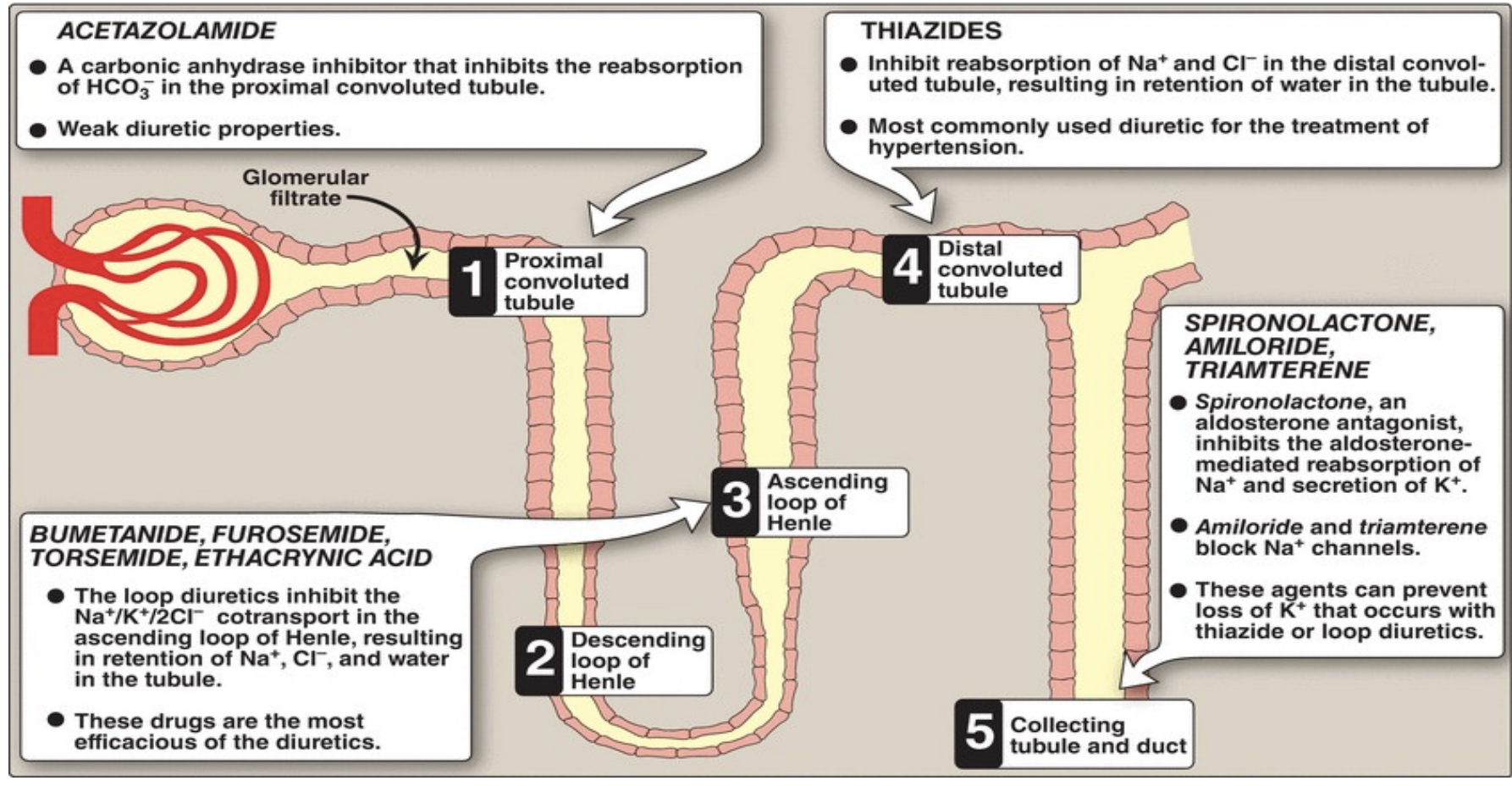
Managing excess electrolytes
duiretics: loss of electrolytes with water via kidneys
insulin activates Na+/K+ pump and shifts potassium in to the cells. need to give glucose at the same time to prevent associated hypoglycaemia – take about 10-20 minutes to work.
Types of fluid replacement and their advantages/ disadvantages
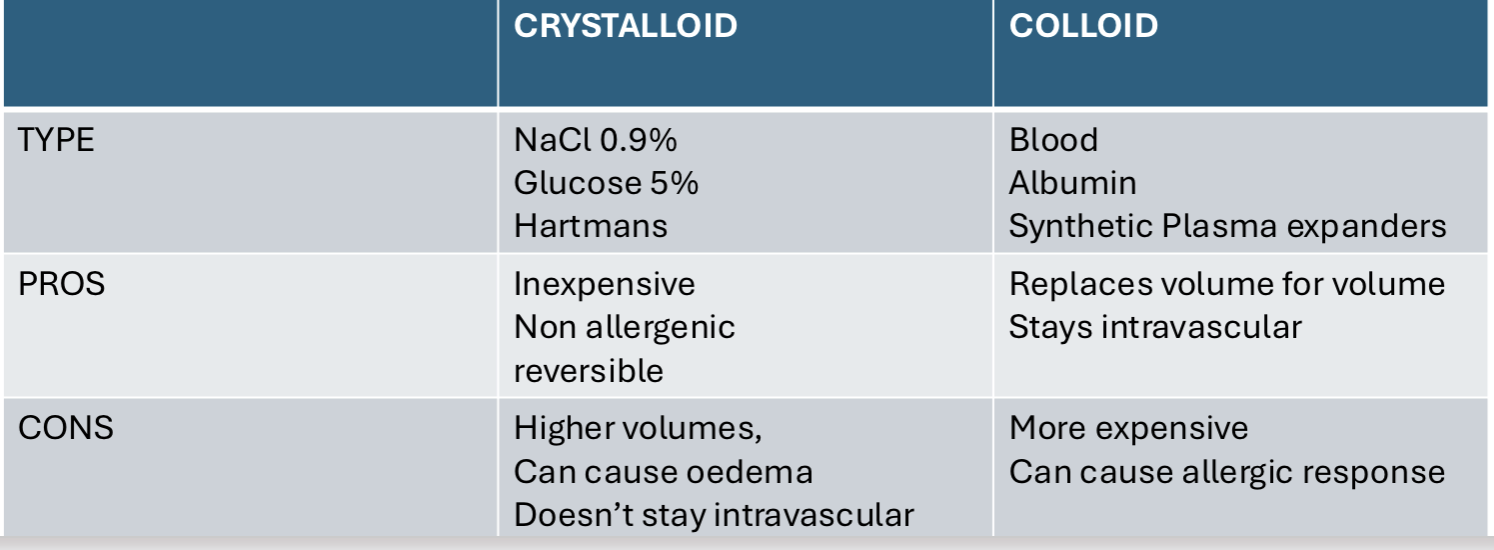
Crystalloid
isotonic (maintain electrolyte balance)
hypotonic (create conc gradient to move electrolytes in to the intravenous space)
Hypertonic (create a conc gradient to move water in to the intravascular space)
Big shifts in fluids
Burns: huge losses of fluid into interstitial space
use of specific formulas - calculations of volume over time
Diabetic Ketoacidosis: extremely high Blood glucose levels
Renal failure
• Peritoneal dialysis
• Uses the peritoneum as the semi-permeable membrane to utilise
osmosis and diffusion of solutes . Dialysate fluid is instilled in to
the peritoneal cavity, and left for a period of time for equilibrium to
occur, then drained off.
• Diffusion occurs both ways, so the constitution of the dialysate
fluid determines amount of fluid and electrolyte shift.