Redox
0.0(0)
0.0(0)
Card Sorting
1/34
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
1
New cards
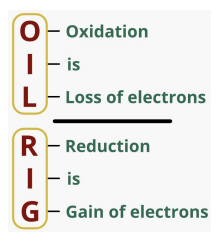
REDOX REACTION
An oxidation-reduction (redox) reaction is a type of chemical
reaction that involves a transfer of electrons between two species.
2
New cards

3
New cards
4
New cards
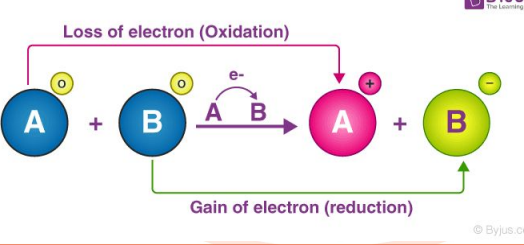
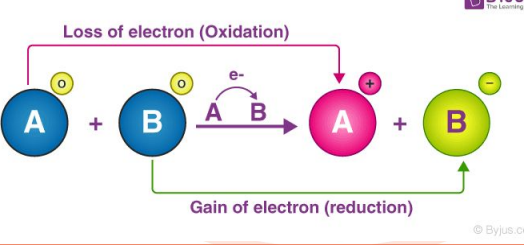
Which atom is
oxidized & which
atom is reduced?
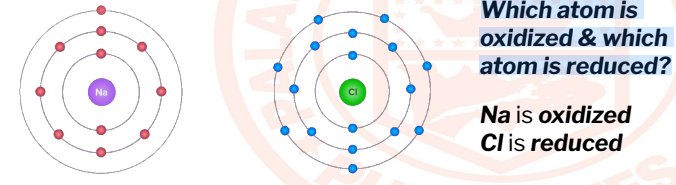
5
New cards
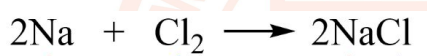
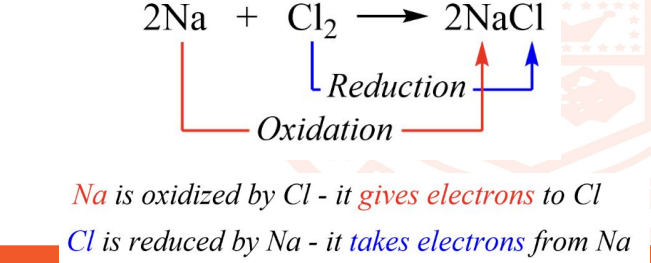
6
New cards
OXIDATION NUMBER
The oxidation state/number of an element corresponds to the number of
electrons, that an atom loses, gains, or appears to use when joining with other
atoms in compounds.
7
New cards
0
8
New cards
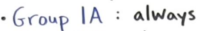

9
New cards
Group 1a

10
New cards
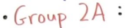

11
New cards
Group 2a

12
New cards

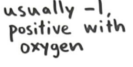
13
New cards
halogens

14
New cards

H

15
New cards
O
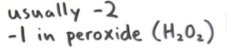
16
New cards
F

17
New cards

0
18
New cards


19
New cards
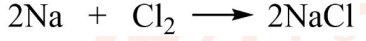
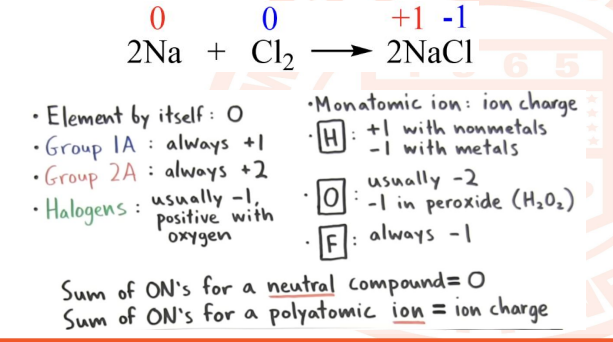
20
New cards


21
New cards

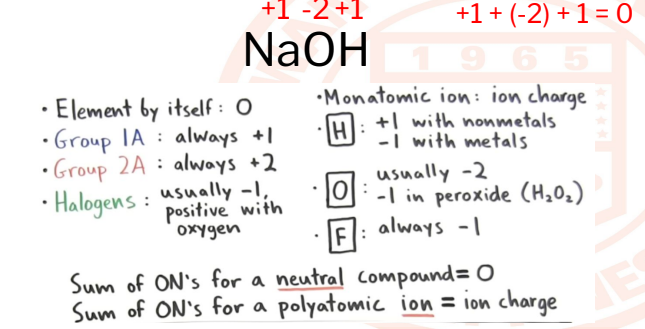
22
New cards

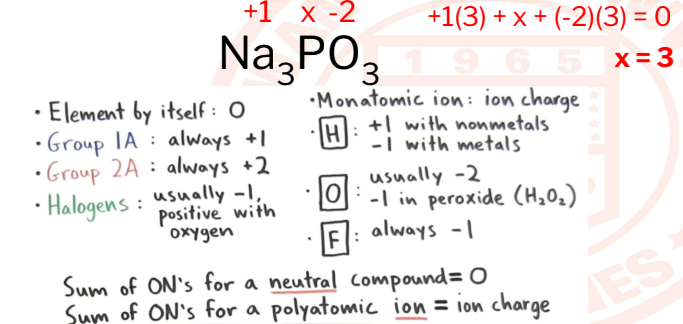
23
New cards

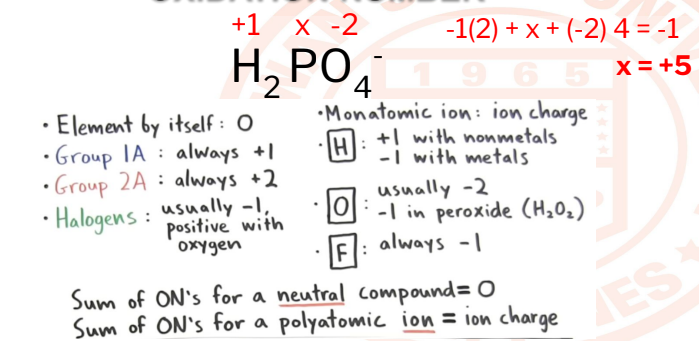
24
New cards
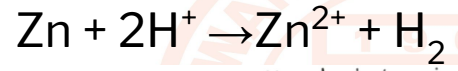
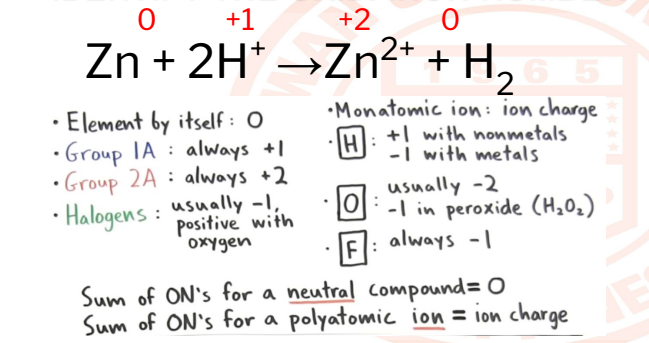
25
New cards
Zn + 2H+ →Zn2+ + H2

26
New cards
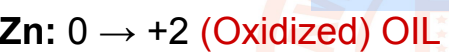
half-reaction:

27
New cards

half-reaction:

28
New cards

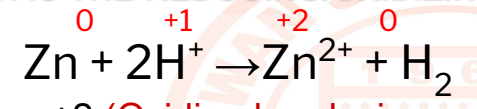
29
New cards


30
New cards


31
New cards
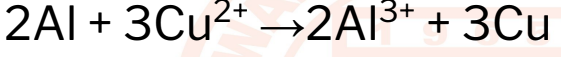

32
New cards

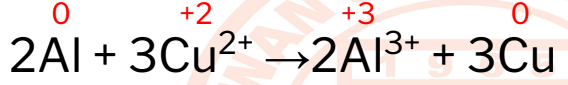
33
New cards

HALF REACTION

34
New cards

HALF REACTION

35
New cards
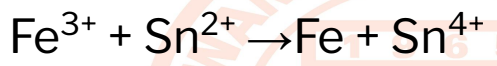
Assign the oxidation numbers to determine which
specie is oxidized and which is reduced, as well as to
identify the reducing and oxidizing agents. Don’t forget
to write the corresponding half-reactions.