Chemistry Unit 7 : Balancing Equations and Chemical Reactions
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
What are the two elements that you should balance last
Hydrogen and oxygen (Oxygen very last)
When balancing what number you you NEVER change
NEVER change the subscript
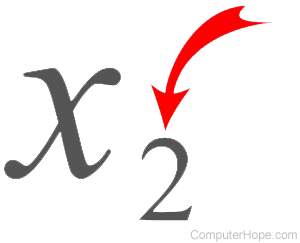
What number goes at the start of a compound that affects ALL elements in that compound?
Coefficients
What number do you NOT write as a Coefficient
One
BALANCE/ fill in Coeficients
_ Mn + _ HI → _ H22 + _ MnI3
2,6,3,2
__ NaCN + __ CuCO3 → __ Na2 CO3 + __ Cu(CN)2
2,1,1,1
Why do chemical reactions happen?
to make the system more stable
What are the two changes that let us know a chemical reaction has occured?
Chemical Change and Physical Change
Define Physical Change
changed to a substances outer appearance (expected color change ex. blue and red making purple)
Define Chemical Change
Changes the composition/ on the molecular and atomic level
EX. temperature change (production of heat)
UNEXPECTED color change (purple and blue making red)
production of gas (bubbles, water vapor, odor)
Production of a precipitate ( solid made with two liquids that falls out solution)
Describe Synthesis reaction
2 or more reactions come together to make one or more (One cation replaces another)
A+B→AB
Describe Decomposition reaction