Biology: All Notes
Unit: 0
Experimentation
Topic 01: Experimental Design Inquiry
At the heart of science is inquiry.
○ ==Inquiry==: The search for information and explanation
Two main steps:
- Making observations
- Forming hypotheses
Recap on making Observations
■ Observations are FACTS.
○ ==Qualitative Data==: Data with descriptions
○ ==Quantitative Data==: Data with numbers/numerical measurements
○ ==Inductive Reasoning==: Derive generalizations based on a large number of specific observations
- (Specific → Generalization)
○ ==Deductive Reasoning==: Specific conclusions are derived from general ideas
- (General Ideas → Specific)
Forming Hypotheses
○ ==Hypothesis==: A prediction that can be tested
■ Results can either support or refute the hypothesis.
■ NEVER SAY “my hypothesis is correct/incorrect”, use the words “support” or “refute”.
Null and Alternative Hypotheses
Always start with a null hypothesis:
| H₀ | A null hypothesis is a hypothesis which the researcher tries to disprove, reject, or nullify
^^Example^^: There is no difference to headache relief between individuals who take Tylenol and those who do not.
After the null, list the alternative hypotheses:
| H₁ | One alternative hypothesis
| H₂ | Two alternative hypotheses
| H₃ | Three alternative hypotheses
^^Example^^:
| H₁ | Tylenol will allow for relief when consumed by people with headaches.
| H₂ | Tylenol will worsen headaches when consumed by patients.
Scientific Method
Most scientific methods do not follow a perfectly structured form.
Scientists can be working with the wrong hypothesis and have to redirect research.
Hypothesis vs Theory vs Law
==Hypothesis==: An explanation that can be tested or disproved
==Theory==: Summarizes major groups of hypotheses
Supported by lots of evidence
Never becomes a law
==Scientific Law==: A statement of fact, usually a mathematical formula
Generally accepted
Description of observation- not “how” or “why”
Experiments
Start with an observation and a hypothesis.
Use Control Groups and Experimental Groups.
Well-designed experiments should include:
- Independent Variable (IV)
- Dependent Variable (DV)
- Control Group (+ and/or -)
- Constants
- The number of trials (3 trials at minimum)
Variables vs Constants
○ ==Independent Variable==: A single factor that is changed by the person in control of the experiment
○ ==Dependent Variable==: The factor which is measured in the experiment
○ ==Constants==: All the factors that stay the same in an experiment
- (Temperature, location, height, weight, etc)
■ Temperature is not always constant
- - -
Experimental Controls
Control Group ⇌ Experimental Group
■ (Control Group = Expected Results) (Experimental Group = Experimental Results)
Control Groups
Controls are essential elements of an experiment:
They help eliminate experimental errors and bias of researchers.
Results of the control experiments validate statistical analysis of the experiment.
■ Controls are NOT constants
Types of Control Groups
There are two types of controls: Positive and Negative
POSITIVE:
- Not exposed to experimental treatment or independent variable, but it IS exposed to a treatment known to produce the expected effect
- ALWAYS have the expected outcome
NEGATIVE:
Not exposed to the experimental treatment or exposed to a treatment that is known to have no effect
Group where nothing is expected to happen
■ Experiments do not need to contain both a positive and negative control
Using Positive Controls
^^Example^^: If there is a new drug to treat headaches, Tylenol can be used as a positive control to show that headaches can be relieved in the patients being tested.
Using Negative Controls
A negative control can be a way of setting a baseline.
■ Ensures no outside variable has affected the results
^^Example^^: If there is a new drug to treat headaches, a placebo can be used as a negative control.
- - -
Introduction to Statistics in AP Biology Statistics
Scientists typically collect data on a sample of a population.
The first step in analysis is to graph and examine the distribution.
Measures of Central Tendencies
○ ==Mean==: The average of the data set
- (x = x+x+x+x+x+x / n)
○ ==Median==: The middle number in a range of data
- (45, 24, 48, 47, 130, 55, 62, 180, 41) → (24, 41, 45, 47, 48, 55, 62, 130, 180) → Median: 48
○ ==Mode==: The value that appears most often
- (2, 4, 4, 4, 5, 7, 7, 8, 9) → Mode: 4
Measure of Variability
○ ==Variability==: The measure of how far a data set diverges from central tendency
○ ==Range==: The difference between largest and smallest values
■ Larger Range → Greater Variability
■ Smaller Ranger → Less Variability
Often used in conjunction with standard deviation.
○ ==Standard Deviation==: A measure of how spread out the data is to the mean
- Low Standard Deviation: Close to the mean
■ Independent variable is most likely causing changes
- High Standard Deviation: The data is farthest from the mean (More spread out)
■ Factors other than the independent variable is causing changes
■ 1 standard deviation from the mean in either direction on the horizontal axis represents 68% of the data
■ 2 standard deviations from the mean in their direction on the horizontal axis represents 95% of the data
■ 3 standard deviations from the mean in either direction on the horizontal axis represents 99% of the data
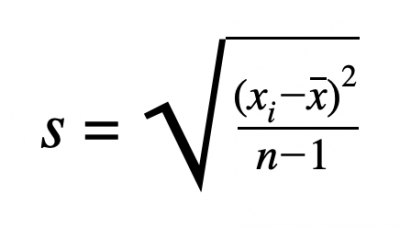
- S = Standard Deviation
- Sigma (Greek Letter) = Sum
- X = Data Point
- X With Line Over It = Mean
- N - 1 = Degrees of Freedom (Number of data values - 1)
■ (8 - 1 = 7)
There are four steps to solve for the standard deviation:
- Find the mean
- Determine the deviation from the mean for each data point and square it
- Calculate the degrees of freedom (N - 1), N is the number data values
- Put it all together and calculate it
Standard Error of the Mean
○ ==Standard Error of the Mean==: Used to determine the precision of confidence in the mean value
■ How well does the mean of the sample represent the true mean of the population
Based On: Standard Deviation (Variability) / Number of Data Points
■ Low standard error: Increase in confidence
■ Commonly given as +/- 2SE SEM (95% confidence)
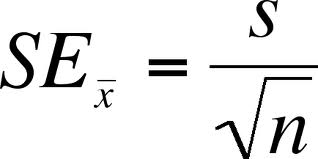
- SE = Standard Error of the Mean
- S = Standard Deviation
- N = Size of Sample
Error Bars
- Overlap = Not significantly different
- Doesn’t Overlap = May be significantly different
- - -
Unit: 1
Topic 1: Structure of Water and Hydrogen Bonding
Chemistry Review
○ ==Matter==: Anything that takes up space and has mass
○ ==Element==: A substance that cannot be broken down
○ ==Compound==: A substance consisting of two or more elements
■ (Combined in a fixed ratio: H₂0)
○ ==Essential Elements==: Elements that are needed to survive and reproduce
■ Essential elements are essential because organisms need them to survive. In the human body, these elements help with bodily function
■ There are 92 naturally occurring elements, and 20-25% are essential
■ CHONP makes up 96% of living matter
↓
Carbon, Hydrogen, Oxygen, Nitrogen, and Phosphorus
○ ==Trace Elements==: Of the 92 naturally occurring elements, these are required by an organism in very small quantities
^^Example^^: Molybdenum - Digestion and excretion, Iron - Oxygen transport, and Chromium - Metabolic function
Chemistry Review
- He - Element Symbol
- 2 - Atomic Number (# of Protons)
- 4.0026 - Atomic Mass / Atomic Number (# of Protons + Neutrons averaged over all isotopes
- Group - Vertical row of elements
■ Same number of valence electrons
- Period - Horizontal row of elements
■ Same number of electron shells
○ ==Bohr Model==: Shows electrons orbiting the nucleus of an atom
■ Electrons are placed on shells around the nucleus
■ Each shell is a different energy level and can hold up to a certain number of electrons
1st Shell - 2e
2nd Shell - 8e
3rd Shell - 18e
○ ==Lewis Dot Model==: Simplified Bohr Model
■ Does not show energy levels
■ Only shows electrons in the valence shell (Outer Shell)
■ Electrons are placed around the symbol
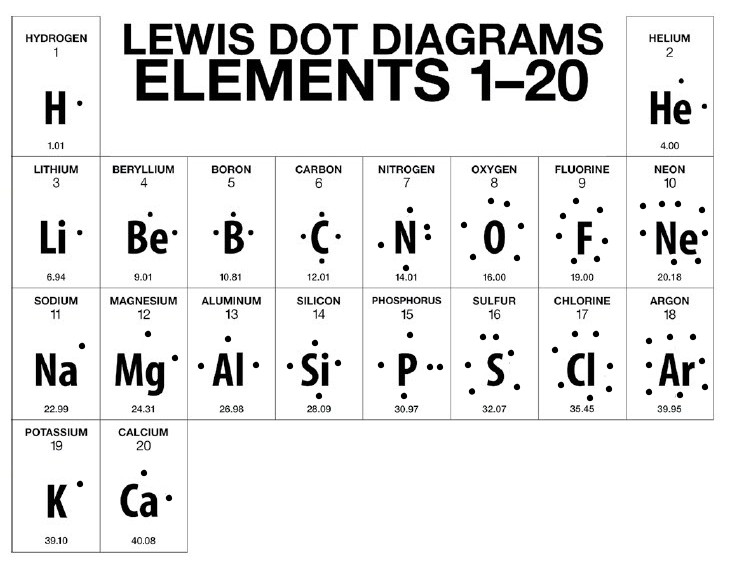
Types of Bonds
- Elements want to be stable
■ Achieve bonds with other elements
○ ==Octet Rule==: Elements will gain, lose, or share electrons to complete their valence shell and become stable (like Noble Gasses)
○ ==Chemical Bonds==: The attraction between two or more atoms, resulting from the sharing or transferring of valence electrons
○ ==Electronegativity==: The measure of an atom’s ability to attract electrons to itself
■ As you go left to right on the periodic table, electronegativity increases
■ As you go down each period on the periodic table, electronegativity decreases
○ ==Covalent Bonds==: Sharing electrons
- Single Bond: 1 pair of electrons
- Double Bond - 2 pairs of electrons
- Triple Bond - 3 pairs of electrons
There are two types of covalent bonds: nonpolar covalent and polar covalent
○ ==Nonpolar Covalent==: Electrons are shared equally between two atoms
○ ==Polar Covalent==: Electrons are not shared equally between two atoms
○ ==Ionic Bonds==: The attraction between oppositely charged atoms (Ions)
Occurs when there is a transfer of electrons from one atom to another atom forming ions
■ Usually between a metal and nonmetal (metal transfers electrons to nonmetal)
■ Forms ionic compounds and salts
○ ==Cation==: Positively charged ion
○ ==Anion==: Negatively charged ion
○==Hydrogen Bonds==: The partially positive hydrogen atom in one polar covalent molecule will attract to an electronegative atom in another polar covalent molecule
○ ==Intermolecular Bond==: Bonds that form between molecules
Why does this happen?
When a hydrogen atom is bonded to an electronegative atom (usually O, N, or F) the electrons are not being shared equally between atoms (this is a polar covalent bond)
■ This causes the hydrogen to have a partial positive charge and the electronegative atom to have a partial negative charge
Properties of Water
- %%Polarity%%: Unequal sharing of the electrons make water a polar molecule
- %%Cohesion%%: Attraction of molecules for other molecules of the same kind
■ Hydrogen bonds between H₂0 molecules hold them together and increase cohesive forces
■ Allows for the transport of water and nutrients against gravity in plants
■ Responsible for surface tension
○ ==Surface Tension==: Property allowing liquid to resist external force
- %%Adhesion%%: The clinging of one molecule to a different molecule
■ Due to the property of H₂0
In plants, this allows water to cling to cell walls to resist the downward pull of gravity
- Capillary Action: The upward movement of water due to the forces of cohesion, adhesion, and surface tension
Occurs when adhesion is greater than cohesion
■ Important for transport of water and nutrients in plants
- Temperature Control
■ ==High Specific Heat==: H₂0 resists changes in temperature
How?
Hydrogen Bonds
■ Heat must be absorbed to break hydrogen bonds, but heat is released when hydrogen bonds form
Importance of High Specific Heat:
- Moderates air temperature
■ Large bodies of water can absorb heat in the daytime and release heat at night
- Stabilizes ocean temperature
■ Benefits marine life
- Organisms can resist changes in their own internal temperature
■ Evaporative Cooling: Water has a high heat of vaporization
■ The molecules with the highest kinetic energy leave as a gas
Importance of Evaporative Cooling:
- Moderates Earth’s climate
- Stabilizes temperature in lakes and ponds
- Prevents terrestrial organisms from overheating (sweating in humans)
- Prevents leaves from becoming too warm in the sun
- Density (Floating Ice): As water solidifies, it expands and becomes less dense
Due to the Hydrogen bonds
■ When cooled, H₂0 molecules move too slowly to break the bonds
■ Allows marine life to survive under floating ice sheets
- Solvent: Dissolving agent in a solution
Water is a versatile solvent
■ Its polar molecules are attracted to ions and other polar molecules. It can form bonds with:
○ ==Solution==: Homogeneous mix of two or more substances
○ ==Solute==: Substance that is dissolved
Like dissolves like
■ Water can interact with sugars or proteins containing many oxygen and hydrogen
■ Water will form hydrogen bonds with the sugar or protein to dissolve it
Ionic Compounds:
- The partially negative oxygen in water will interact with a positive atom
- The partially positive hydrogen in water will interact with a negative atom
- Dissolves ions
- - -
Topic 2: Elements of Life
Carbon
○ ==Organic Chemistry==: The study of compound with covalent-bonded carbon
○ ==Organic Compounds==: Compounds that contain carbon and hydrogen atoms
■ Has four valence electrons
Carbon can form single, double, or triple covalent bonds
■ Commonly formed with hydrogen, oxygen, and nitrogen
The type and number of covalent bonds carbon forms with other atoms affects the length of the carbon chain and shape of the molecule
Carbon Chains
Carbon can use its valence electrons to form covalent bonds to other carbons
○ ==Hydrocarbons==: Molecules made of carbon and hydrogen atoms
Carbon chains form the skeletons of most organic molecules
Skeletons can vary in:
- Link
- Branching
- Double bond positions
- Presence of rings
○ ==Functional Groups==: Chemical groups attached to the carbon skeleton that participate in chemical reactions
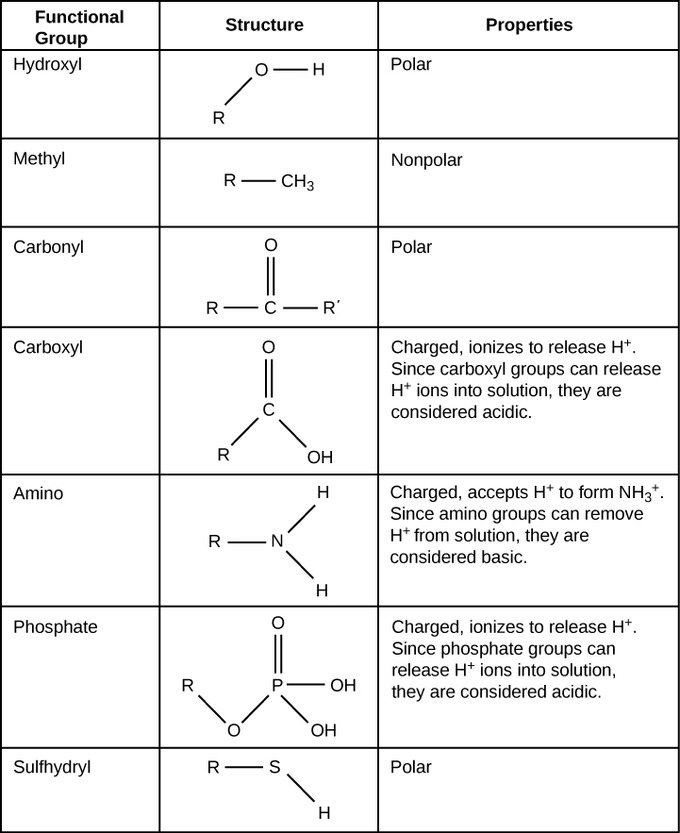
Topic 3: Introduction to Biological Macromolecules: Molecular Diversity Due to Carbon
Variations in carbon skeletons allows for molecular diversity
Carbon can form large molecules known as macromolecules
Four classes of macromolecules (molecules made of smaller subunits):
- Carbohydrates
- Proteins
- Lipids
- Nucleic Acid
■ Carbohydrates, Proteins, and Nucleic Acids are polymers
Formation and Breakdown of Macromolecules
○ ==Polymers==: Chain-like macromolecules of similar or identical repeating units that are bound by covalent bonds
○ ==Monomers==: The repeating units that make up polymer groups
○ ==Dehydration Synthesis==: Bonds two monomers with the loss of H₂O
○ ==Hydrolysis==: Breaks the bonds in a polymer by adding H₂O
- - -
Carbohydrates
Includes sugar and polymers of sugars
Contain a Carbonyl group and a hydroxyl group
Elements: Carbon, Hydrogen, and Oxygen (Ratio - 1:2:1)
○ ==Monosaccharides==: Simple sugars
■ Most common is Glucose
■ Nutrients and fuel (energy) for cells
■ Can serve as building blocks for amino acids
○ ==Disaccharides==: Two monosaccharides joined together by covalent bonds
■ Most common is Sucrose
■ Monomers of Sucrose: Glucose and Fructose
○ ==Polysaccharides==: Many sugars
Storage Polysaccharides
In plants, Starch is used for storage (polymer of glucose monomers)
Animals store glycogen (polymer of glucose)
Structural Polysaccharides
Cellulose is a tough substance that forms plant cell walls
Chiten forms exoskeleton
Lipids
○ ==Lipids==: Class of molecules that do not include true polymers
■ Generally small
■ Lipids are nonpolar hydrophobic
Types of Lipids: Fats, Phospholipids, Steroids, and Oils
■ Lipids are also Carbon, Hydrogen, and Oxygen, but not in a 1:2:1 ratio
Fats
Fats are composed of Glycerol and Fatty Acids
○ ==Glycerol==: Alcohol (Hydroxyl Group)
○ ==Fatty Acids==: Long chains of Carbon (Carboxyl Group at one end)
3 Fatty Acids join to a Glycerol by ester linkage
■ Bonds between a hydroxyl and carboxyl group
Classified as a saturated fatty acid or an unsaturated fatty acid
○ ==Saturated Fatty Acid==: No double bonds between Carbons in the Carbon chain
■ More Hydrogen
○ ==Unsaturated Fatty Acid==: Contains one or more double bonds
Phospholipids
Major components of cell membranes
■ Two Fatty Acids attached to a Glycerol and a Phosphate
■ Assemble as a bilayer in H₂O
■ Tails are hydrophobic
■ Head is hydrophilic
Steroids
■ Lipids that have four fused rings
■ Unique groups attached to the ring determine the type of Steroid
^^Example^^: Testosterone
- - -
Proteins:
Starts as an amino acid → peptide → polypeptide → protein
○ ==Protein==: Molecules consisting of polypeptides (polymers of amino acids)
Elements: Carbon, hydrogen, oxygen, nitrogen, and sulfur
What does the shape determine? → function
==Amino Acids==: Molecules that have an amino group and a carboxyl group
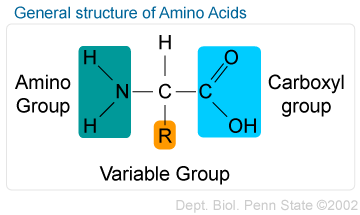
^^Examples^^: Alanine and Glycine
Each amino acid (AA) has a unique side chain
■ Side chains can be grouped as: nonpolar (hydrophobic), polar (hydrophilic), and charged/ionic (hydrophilic)
■ Side chains interact, which determine the shape and function of the protein
Formation of Peptide Bonds
To form a peptide bond, the carboxyl group of one AA must be positioned next to the amino group of another AA
Polypeptides
○ ==Polypeptides==: Many AA linked by peptide bonds
Each end is chemically unique:
- One end is a free amino group (N-Terminus)
- One end is a free carboxyl group (C-Terminus)
The sequence of AA's determines the 3D shape
When a polypeptide twists and folds (because of R group interaction) it forms a protein
REMEMBER:
Function of proteins include:
- %%Antibody%%: Helps protect the body from disease
- %%Enzyme%%: Carry out chemical reactions or assist in creating new molecules
- %%Messenger%%: Transmits signals (hormones)
- %%Structural%%: Provides structure and support
- %%Transport/Storage%%: Bind to and carry small molecules and atoms through the body (hemoglobin)
Levels of Protein Structure:
- %%Primary%% → Liner chain of AA
- %%Secondary%% → Coils and folds due to hydrogen bonding within the polypeptide backbone
- %%Tertiary%% → 3D forming because of side chains
- %%Quaternary%% → Association of two or more polypeptides
Nucleic Acids
○ ==Nucleic Acids==: Polymers made of nucleotide
Elements: Carbon, hydrogen, oxygen, nitrogen, and phosphorus
Function to: store, transmit, and express hereditary information
Two forms: DNA and RNA
Components of nucleic acids
Contain 3 parts: Phosphate group, 5-carbon sugar (pentose), and nitrogenous base
Nitrogenous Base
In DNA: Guanine, Cytosine, Thymine, and Adenine
In RNA: Guanine, Cytosine, Uracil, and Adenine
Two types
Pyrimidines: Single Ring
Purines: Double Ring
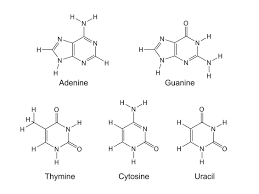
Five Carbon Sugar
DNA: Deoxyribose
RNA: Ribose
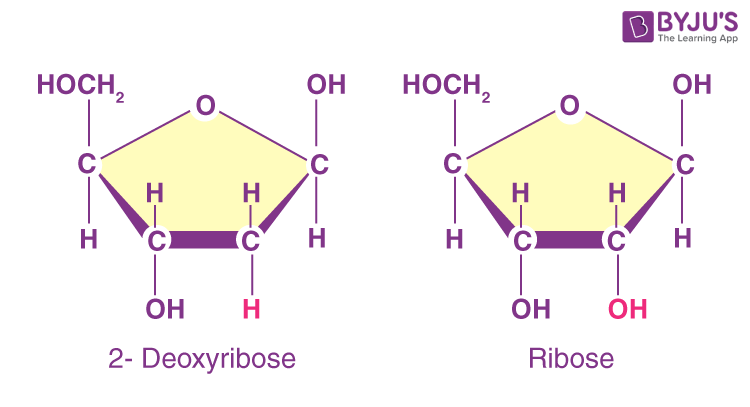
Polynucleotides
Phosphate groups link adjacent nucleotide
What do you call this linkage? → Phosphodiester linkage
Directionality? → 5’ to 3''
The sequence of bases along the DNA or mRNA is unique for each gene
What does it dictate? → Dictates AA sequences and structure
| Macromolecule | Elements Involved | Monomer (Sub-unit) | Polymer |
|---|---|---|---|
| Carbohydrates | Carbon, hydrogen, and oxygen | Monosaccharides | Polysaccharides |
| Proteins | Carbon, hydrogen, oxygen, nitrogen, and sulfur | Amino Acids | Polypeptides |
| Lipids | Carbon, hydrogen, and oxygen (phosphorus for phospholipids) | Glycerol and Fatty Acids | Does not contain true polymers |
| Nucleic Acids | Carbon, hydrogen, oxygen, nitrogen, and phosphorus | Nucleotide | DNA and RNA |
- - -
Unit: 2
Unit 2 Day 1: Studying Cells in a Laboratory Setting and Cell Types Overview:
Cell Theory:
- All organisms are made of cells
- The cell is the smallest unit of life
- All cells come from pre-existing cells
■ Remember….cell structure is correlated to cellular function
Studying Cells in a Lab
Microscopy
Why do scientists use microscopes? → To visualize cells too small to see with the naked eye and study their complexity
Types of Microscopes
Light Microscope (LM) - Visible light passes through a specimen and then through glass lesnses, which magnify the image
Image quality depends on…
○ ==Magnification==: The ratio of an objects image size to its real size
○ ==Resolution==: The measure of the clarity of the image
○ ==Contrast==: Visible differences in parts of the sample
■ LMs can magnify effectively to about 1,000 times
What are the limitations of LMs? → Organelles are too small to be resolved (focused) by an LM
Electron Microscopes (EMs)
Types:
Scanning Electron Microscopes (SEMs) - Focus a beam of electrons onto the surface of a specimen, proving images that look 3D
Transmission Electron Microscopes (TEMs) - Used to study the internal structure of a cell
What are the limitations of EMs? → Cannot observe living cells
Cell Fractionation
What does cell fractionation do? → Takes cells apart and separates the major organelles from one another
What tool is used to seperate cells into their component parts? → Ultracentrifuge
Cell Types
■ There are two types of cells: prokaryotes and eukaryotes
| Prokaryotic Cells | All Cells | Eukaryotic Cells |
|---|---|---|
| No nucleus | Are bound by a plasma membrane | DNA in a nucleus that is bound by a membranous nuclear envelope |
| DNA in an unbound region called the nucleoid | Contain Cytosol (Cytoplasm) | Membrane-bound organelles |
| No membrane-bound organelles | Contain chromosomes (carry genes) | Larger than Prokaryotic Cells |
| Typically small | Contain ribosomes (make proteins) | Multicellular |
| Unicellular | ||
| Ex: Bacteria | Ex: Animals, plants, fungi, and protists |
What does compartmentalization do? → Allows for different reactions in different locations
What does it prevent? → Prevents interfering reactions from occurring in the same locations
- - -
The Endomembrane system
■ The Endomembrane System regulates protein traffic and performs metabolic functions
Components of the endomembrane system:
- Nuclear envelope
- Endoplasmic Reticulum (ER)
- Golgi Apparatus
- Lysosomes
- Vacuoles
- Plasma Membrane
How are these components connected? → They are connected continuously (already connected) or are connected via the transfer of vesicles
The eukaryotic cell’s genetic instructions are housed in the nucleus and carried out by the ribosomes
Nucleus
Contains chromosomes
Enclosed in a nuclear envelope
Double Membrane
Has pores
Regulates entry and exit of materials
Contains a nucleolus
Nucleolus is a dense region of the nucleus that makes ribosomes
Exits via nuclear pores
How are ribosomes formed? → rRNA and proteins make ribosomes
What do ribosomes do with the mRNA message? → They translate message to the primary structure of proteins (Amino Acids)
Ribosomes
Function: Make proteins
Locations:
Cytosol: Free ribosomes are located in the Cytosol (Cytoplasm)
Bound on the outside of the endoplasmic reticulum or the nuclear envelope
Endoplasmic Reticulum
■ A network of membranous sacs and tubes
Functions:
- Makes membranes
- Compartmentalize (separates) the cell to keep proteins formed in the rough Er seperate from those of free ribosomes
- Two Types: Rough Endoplasmic Reticulum and Smooth Endoplasmic Reticulum
Smooth ER
- Lacks ribosomes
- Makes lipids
- Metabolizes carbohydrates (Breaks Carbs down)
- Detoxifies poison
- Stores calcium
Rough ER
Has bound ribosomes which secrete glycoproteins
Glycoproteins are proteins bonded to carbohydrates
Distributes transport vesicles, proteins surrounded by membranes
Golgi Apparatus
■ Consists of flatted membranous sacs called Golgi Apparatus
- Has directionality
Cis Face: Receives vesicles from the ER
- Faces ER
Trans Face: Sends vesicles back out into the cytosol to other locations or to the plasma membrane for secretion
- Faces plasma membrane
Functions:
Receives transport vesicles with materials from the ER
Modifies materials
Sorts materials
Adds molecular tag
Packages materials into new transport vehicles that exit the membrane via exocytosis
(Exo - Exit, Cytosis - Cell)
Lysosomes
■ Membranous sacs of hydrolytic enzymes that digest macromolecules
Function:
- Can hydrolyze proteins, fats, polysaccharides, and nucleic acid
○ ==Phagocytosis==: Cellular eating (A cell engulfs another cell)
○ ==Autophagy==: Cells eat itself for renewal (Breaks down own components)
Peroxisomes
Similar to lysosomes
Membrane bound metabolic component
Catalyze reactions that produce H²O² (Hydrogen Peroxide)
Enzymes in peroxisomes then break down H²O² to water
Vacuoles
■ Large vesicles that stem from the ER and Golgi
Types:
- %%Food Vacuole%%: Formed by phagocytosis and digested by lysosomes
- %%Contractile Vacuole%%: Pump excess water out of cells
- Found in freshwater protists
- %%Central Vacuole%%: Hold inorganic ions and water
- Found in many mature plant cells
- Important for turgor pressure (Holds water in plant cells)
- - -Energy Organelles
○ ==Endosymboint Theory==: The theory that explains the similarities mitochondria and chloroplasts have to a prokaryotes
■ Theory that states an early eukaryotic cell engulfed a prokaryotic cell
■ Prokaryotic cell became an endosyboint (cell that lives in another cell)
Evidence:
- Double membrane
- Ribosomes
- Circular DNA
- Capable of functioning on their own
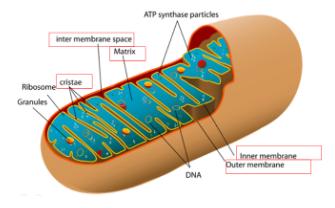
Mitochondria
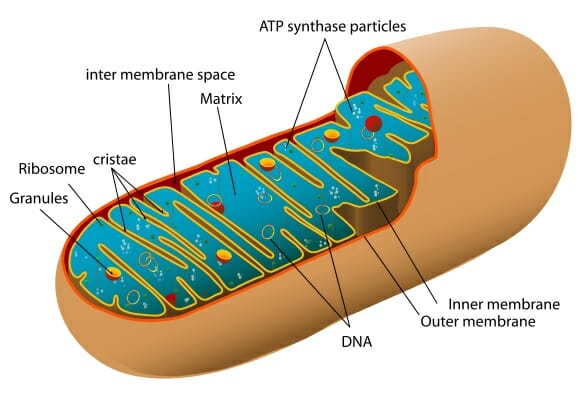
Function: Site of cellular respiration, a metabolic process that generates ATP energy
■ ATP Energy = Usable Energy
Structure of the double membrane: Has a smooth outer membrane and inner membrane is folded into Cristae (<------ The electron transport chain)
%%Intermembrane%%: Space between inner and outer membrane
%%Mitochondrial Matrix%%: Enclosed by the inner membrane
Location for the Krebs Cycle
Contains:
- Enzymes that catalyze cellular respiration and ATP energy
- Mitochondrial DNA
- Ribosomes
■ The number of mitochondria in a cell correlated with metabolic activity
- Cells with high metabolic activity have more mitochondria
^^Ex^^: Cells that move/contract (Muscles in humans)
Chloroplasts
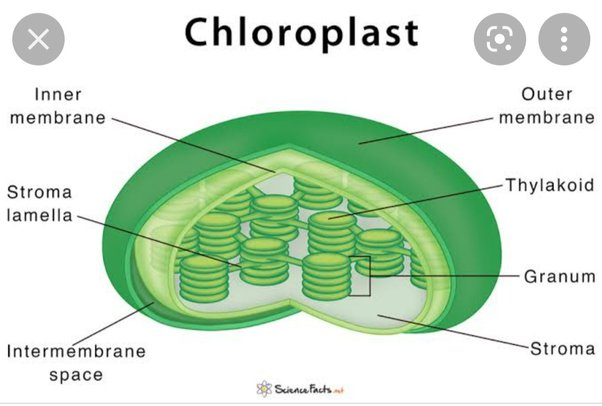
Found in the leaves of plants and algae, are the sites of photosynthesis
- %%Chlorophyll%%: Green pigment in plants (absorbs light energy)
Inside of its double membrane:
- %%Thylakoids%%
- Membranous sacs that can organize into stacks called Granum
- %%Stroma%%: The internal fluid
- Calvin Cycle occurs here
- - -
The Cytoskeleton
The Cytoskeleton
■ A network of fibers throughout the cytoplasm
Functions: Structural support (shape) and mechanical support (movement)
There are three types of fibers in the cytoskeleton:
- %%Microtubules%% (Thickets of the three)
- %%Microfilaments%% (Thin and skinny)
- %%Intermediate Filaments%% (Smaller than Microtubules, but larger than Microfilaments)
Microtubules:
Functions:
- Shapes cell (structural support)
- Guides movements of organelles (Think: Tracks)
- Separates choromosomes during cell division
- Cell motility (i.s. Cilia and Flagellum)
Centrosomes and Centrioles
- The centriole is a “microtubule-organizing center”
- Separates chromosomes in animal cells
- Microtubules grow out from a centrosome
■ Only found in animal cells
Cilia and Flagella
- Microtubules control beating of Cilia and Flagella
- Cilia and Flagella have different beating patterns
Common Structure:
- Core of microtubules
- Anchored by a “basel body” (Thing attached to the cell membrane)
Dynein is a motor protein that drives the movements of cilium and flagellum
Microfilaments (Actin Filaments)
Functions:
- Maintains cell shape
- Bear tension
- Forms a 3D network called cortex (Supports shape)
- Assists in muscle contraction and cell motility
- Actin works with myosin to cause contraction
- Division of animal cells
○ ==Pseudopodia==: Cellular extension of amoeba
○ ==Cytoplasmic Streaming==: Circular flow of cytoplasm within cells
Intermediate Filaments:
Functions:
- Maintains cell shape
- Anchor nucleus and organelles
- Form the nuclear Lamina
- Lines the Nuclear Envelope
- - -
Cell Size
Cell Size
Cellular metabolism depends on cell size
Metabolism - How they break apart
■ Cell waste must leave
■ Dissipate thermal energy
■ Nutrients and other resources (chemical materials) must enter
At a certain size, it begins to be too difficult for a cell to regulate what comes in and what goes out of the plasma membrane
Surface Area to Volume
The size of a cell will dictate the function
Cells need a high surface area-to-volume ratio to optimize the exchange of material through the plasma membrane
Formulas
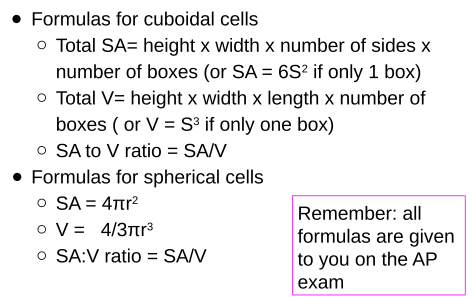
Example: Cubiodal Cell
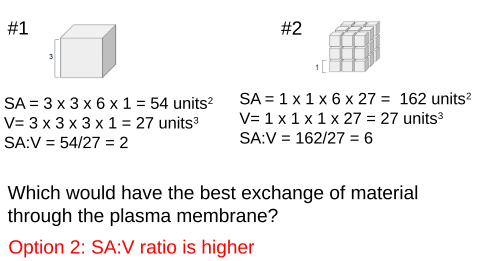
Example: Spherical Cell
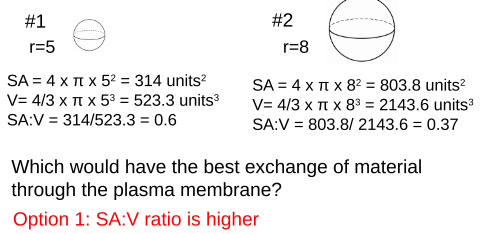
Eukaryotic cells can reach above 100nm (nanometer) in diameter, while prokaryotic cells, like bacteria, remain small, reaching only to about 10nm in diameter. How are eukaryotic cells able to become so much larger than prokaryotic cells while still efficiently exchanging material through the plasma membrane?
What does this mean for a cell? →
Cells tend to be small
- Small cells have a high SA:V ratio
- Optimizes exchange of materials at the plasma membrane
- Larger cells have a lower SA:V ratio
- Lose efficiency exchanging materials
■ The cellular demand for resources increases
■ Rate of heat exchange decreases
- - -
Plasma Membrane and Membrane Permeability
Phospholipid Bilayer Structure
Phospholipid Bilayer: A double layer of phospholipids
■ Phospholipids are amphipathic molecules
○ ^^Amphipathic^^: Has both a hydrophobic region and a hydrophilic region
Examine the phospholipid to the right and identify the structures:
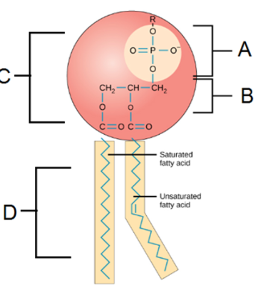
A: Phosphate Group
B: Glycerol
C: Hydrophilic Head
D: Hydrophobic Tails
■ A bilayer forms because they are amphipathic
■ Hydrophilic heads orient toward aqueous environments
■ Hydrophobic tails are facing inwards away from aqueous environments
Fluid Mosaic Model
A model to describe the structure of cell membranes
○ ^^Fluidity^^: Phospholipids are capable of movement
○ ^^Mosaic^^: Comprised of many macromolecules
Phospholipids
Cholesterol
Proteins
Carbohydrates
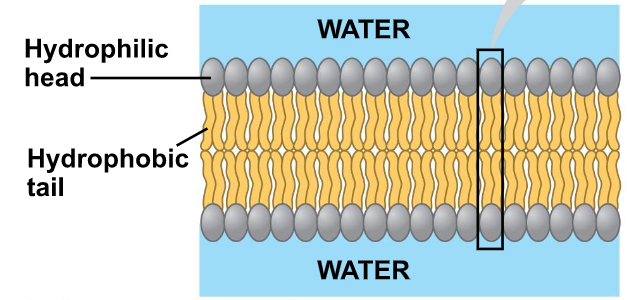
The fluidity of Membranes
○ ^^Fluid^^: Membrane is held together by weak hydrophobic interactions so phospholipids can move and shift
Which way do lipids drift? → Laterally/Rarely flip-flops transversely (Across the membrane)
Effects of Temperature on Fluidity
As temperatures cool, membranes switch from a fluid to a solid state
Which types of fatty acids are more fluid? Why? → Unsaturated fatty acids are more fluid than saturated fats due to kinks
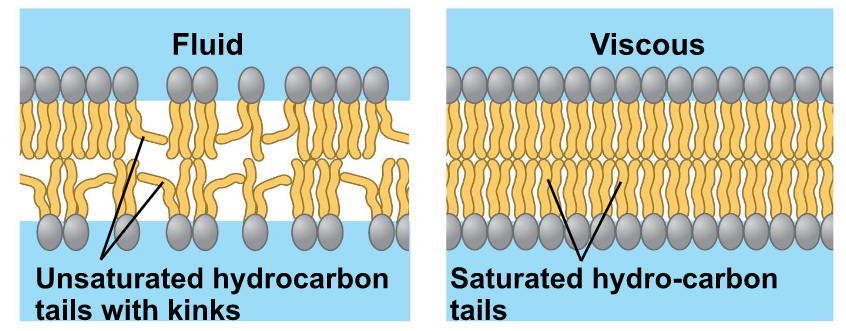
Cholesterol has different effects on membrane fluidity at different temperatures
- High Temp: Reduces movements
- Low Temp: Maintains fluidity by preventing tight packing
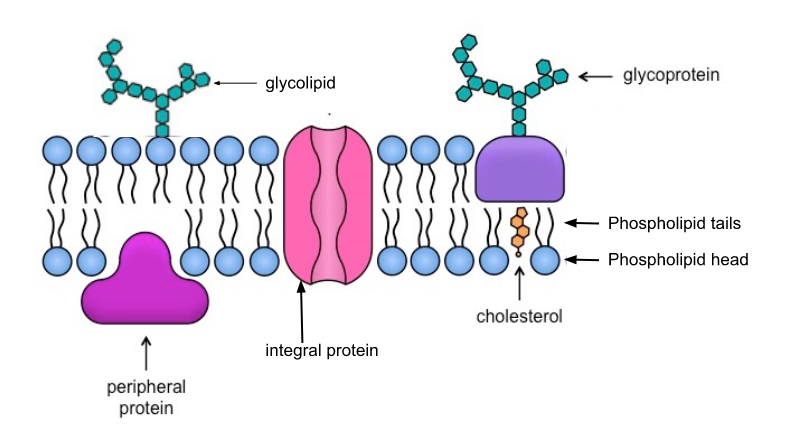
Membrane Proteins and Their Functions
Proteins determine most of the membrane’s specific functions
Categories:
- Integral proteins
- Embedded in the lipid bilayer
- Transmembrane proteins (spans the membrane)
- Amphipathic
- Peripheral Proteins
- Bound to the surface of the membrane
Membrane Carbohydrates
Important for cell-to-cell recognition
- Glycolipids
- Carbohydrates bonded to lipids
- Glycoproteins
- Carbohydrates bonded to proteins
- Most abundant
Draw and Label the cell membrane:
Membrane Functions
- Transport
- Enzymatic Activity
- Signal Transductions
- Cell-cell Recognition
- Intercellular Joining
- Attachment to the cytoskeleton and extracellular matrix (ECM)
Transport
A cell must exchange materials with its surroundings, a process controlled by the plasma membrane
○ ^^Selectively Permeable^^: Selectively letting some things pass through, but not others (like a screen)
Which types of molecules can pass through easily and rapidly? → Small hydrophobic (nonpolar) molecules
Which types of molecules cannot pass through easily and require assistance? → Large, hydrophilic (polar) molecules
Transport Proteins
Are transport proteins integral or peripheral? Integral
○ ^^Channel Proteins^^: Has a hydrophilic channel that is used as a tunnel
Ex: Aquaporins
○ ^^Carrier Proteins^^: Bind to molecules and change shape to shuttle them across the membrane
A transport protein is specific for the substance it moves
Plant Cell Barriers
Plants have a cell wall that covers their plasma membranes
Extracellular structure (not found in animal cells)
■ Made of cellulose
Provides:
- Shape/Structure
- Protection
- Regulation of water intake
Communication
Plants communicate through plasmodesmata
○ ^^Plasmodesmata^^: Hole-like structures in the cell wall filled with cytosol that connect adjacent cells
- - -
Passive Transport
Selective Permeability
Some substances can cross the membrane more easily than others
Easy passage across the membrane: small, hydrophobic (nonpolar) molecules
Difficult passage or protein assisted passage: Large hydrophilic (polar) molecules + ions
Transport Across a Membrane
There are two types of transport across a membrane: passive and active
○ ==Passive==: Without the use of energy
○ ==Active==: Uses energy
Passive Transport
Passive Transport:
■ Molecules move down their concentration gradient from high to low concentration
Diffusion:
○ ==Diffusion==: Molecules spread out evenly in the available space
■ Substances move from a high to a low concentration
■ Move with the concentration gradient
Dynamic Equilibrium:
■ Molecules diffuse directly across the membrane
■ Different rates of diffusion for different molecules
What factors impact the rate of diffusion? → Concentration gradient, distance, area, and the presence of transport proteins
Osmosis:
○ ==Osmosis==: The diffusion of water across a selectively permeable membrane
■ Osmosis can happen without a protein
■ Water diffused from a region of lower solute (high water) concentration to a region of higher solute (low water) concentration
Facilitated Diffusion:
==Facilitated Diffusion==: To help
■ Speeds up the rate of diffusion of: small ions, water, and carbohydrates
■ Two categories of transport proteins: channel and carrier
■ Each transport protein is specific for substances it can facilitate movement for
Channel Proteins:
==Channel Proteins==: Provides channels to allow specific molecules or ions to cross the membrane
■ Channel is hydrophilic
Which type of molecules pass through channel proteins? → Small polar molecules and ions
○ ==Aquaporins==: Proteins used for the facilitated diffusion of water
○ ==Gated channels==: Only open and close in response to stimulus
Carrier Proteins:
==Carrier Proteins==: Change shape that translocates the solute-binding site across the membrane
Which type of molecules pass through carrier proteins? → Lipid insoluble molecules
- - -
Active Transport
Comparing Passive and Active Transport that Involve Membrane Proteins
Facilitated Diffusion = Passive (no energy)
■ High to low concentration
Active Diffusion = Requires energy
■ Low to high concentration
○ ==Active Transport==: Transport of molecules that require energy because it moves a solute against its concentration gradient
EX: Pumps, cotransports, exocytosis, and endocytosis
Types of Energy:
Cells can use various forms of energy to drive active transport against concentration or charge gradients
■ Fueled by the energy of ATP
How is energy released? → ATP hydrolysis
■ Membrane potential can drive the transport of charged molecules
■ Light energy
Pumps:
Active transport that allows cells to maintain concentration gradients that differ from their surroundings
○ ==Pumps==: Maintain membrane potential
○ ==Membrane Potential==: Unequal concentrations of ions across the membrane results in an electric charge (electrochemical gradient)
■ The cytoplasm is relatively negative in comparison to the extracellular fluid
■ Hydrogen is stored in electrochemical gradients
○ ==Electrochemical Gradient==:Two combined forces
■ A chemical force = Ions concentration gradient
■ An electrical force = The effect of the membrane potential on the ion’s movements
Examples of Pumps:
○ ==Electronegative Pumps==: Proteins that generate voltage across the membrane
■ Can be used as an energy source for cellular processes
Sodium Potassium Pump
■ Animal cells will regulate their relative concentrations of Na+ and K+
■ 3 Na+ get pumped out of the cell
■ 2 K+ get pumped into the cell
- Results in a +1 net charge to the extracellular fluid
Proton Pump
○ ==Proton Pump==: Integral membrane protein that builds up proton gradient
Used by: Plants, fungi, and bacteria
■ Pumps H+ out of the cell
Cotransport
○ ==Cotransport==: Active transports of solute indirectly drives transport of another solute
■ Uses the energy stored in electrochemical gradients (generated by pumps) to move substances against their concentration gradient
■ Plants use cotransport for sugars and amino acids
Example:
- Sucrose can travel into a plant cell against its concentration gradient ONLY if it is coupled with H+ that is diffusing down its electrochemical gradient
Bulk Transport
Large molecules, such as polysaccharides and proteins, cannot enter or leave through the lipid bilayer independently nor with the use of a transport protein
- How do they cross the membrane?→Bulk via vesicles
- Bulk transport requires energy
- EX: Exocytosis, Endocytosis
Exocytosis
○ ==Exocytosis==: Transport vesicles migrate, fuse, and release their contents with the membrane
■ Many secretory cells use exocytosis to export their products
EX: Nerve cells release neurotransmitters
Endocytosis
○ ==Endocytosis==: The cell takes in macromolecules by forming vesicles from the plasma membrane
Types:
○ ==Phagocytosis==: Cellular eating
■ Cell engulfs a particle in a vacuole to be later digested by a lysosome
○ ==Pinocytosis==: Drinking
■ Molecules are taken up when extracellular fluid containing dissolved molecules is "gulped”into tiny vesicles
○ ==Receptor mediated endocytosis==: Binding of ligands to receptors triggers vesicle formation
○ ==Ligands==: Any molecule that binds to a receptor site of another molecules
- - -
Tonicity and Osmoregulation
Water Balance
○ ==Tonicity==: The ability of an extracellular solution to cause a cell to gain or lose water
What does it depend on? → The concentration of solute that cannot pass through the cell membrane
Types of solutions:
- Isotonic
- Hypertonic
- Hypotonic
○ ==Osmoregulation==: Cells must be able to regulate their solute concentrations and maintain water molecules
Isotonic Solutions
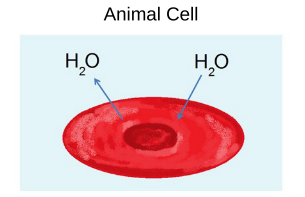
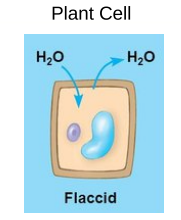
The concentration on nonpenetrating solutes inside the cell is equal to that outside the cell
How does water move? → Water moves in and out of the cell at the same rate
Hypertonic Solutions
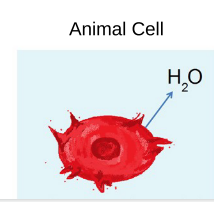
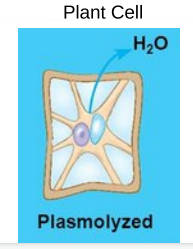
The concentration of nonpenetrating solutes is higher outside the cell
How does water move? → Water moves outside the cell to the extracellular fluid
○ ==Plasmolysis==: Vacuole shrinks and the plasma membrane pulls away from the cell wall
Hypotonic Solutions
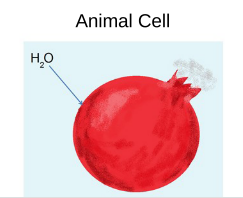
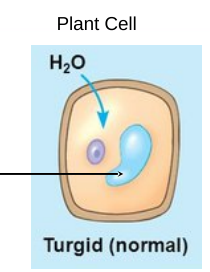
The concentration of nonpenetrating solutes is lower outside of the cell
■ Animal cells swell and lyse
■ Plant cells work optimally
How does water move? → Water moves inside the cell
Water Potential
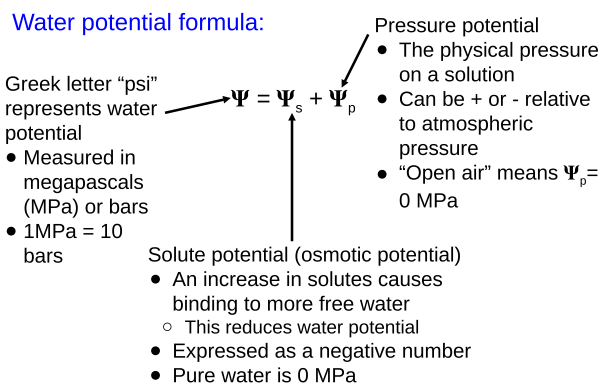
○ ==Water Potential==: A physical property that predicts the direction water will flow
Water will flow from areas of:
- High water potential to areas of low water potential
- Low solute to areas of high solute concentrations
- High pressure to areas of low pressure
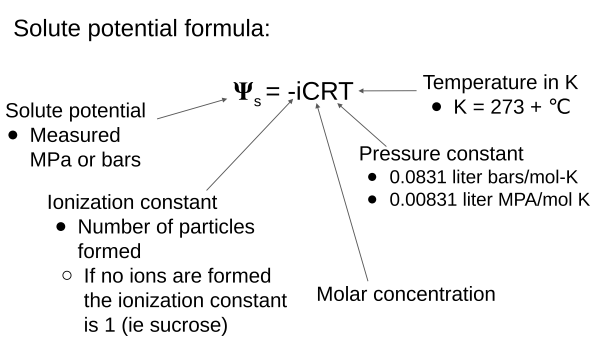
Water Potential Formula:
Solute Potential Formula:
- - -
Unit 3
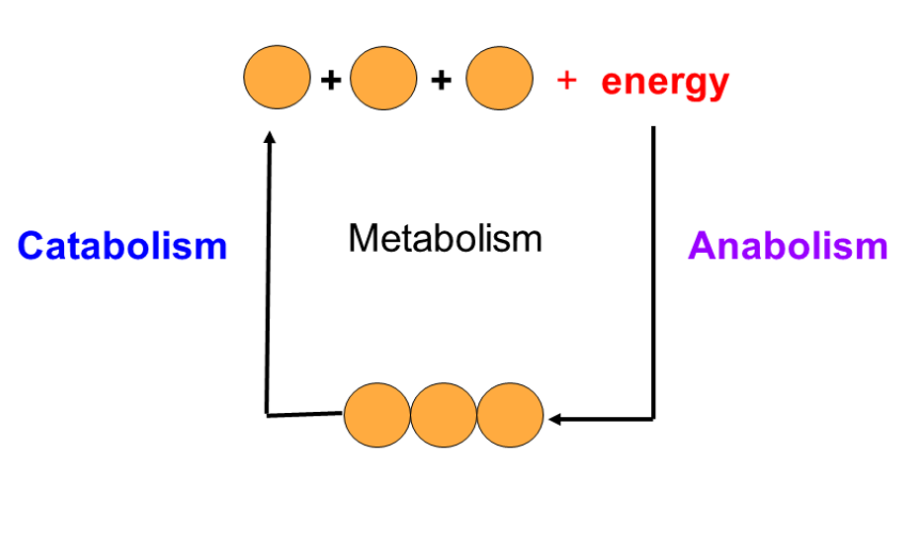
Metabolism
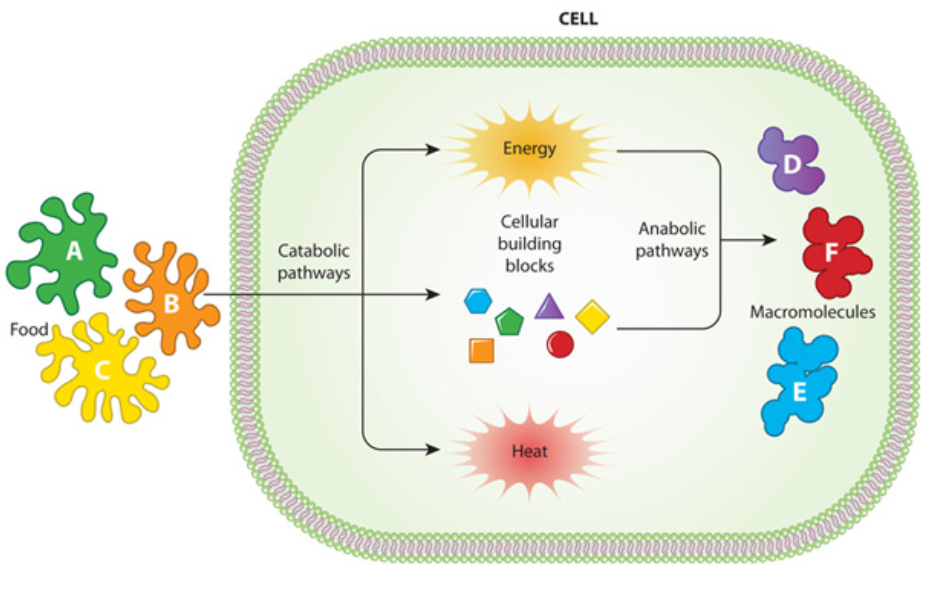
○ ==Metabolism==: All of the chemical reactions in an organism
Chemical Reactions:
○ ==Reactants==: Substances that go into a chemical reaction
○ ==Products==: Substances that are released from a chemical reaction
==EX==: 2H₂+O₂ → 2H₂O
2H₂+O₂ = Reactants
2H₂O₂ = Products
○ ==Metabolic Pathways==: A series of chemical reactions that either build up or break down complex molecules
Each step is being catalyzed by a specific enzyme
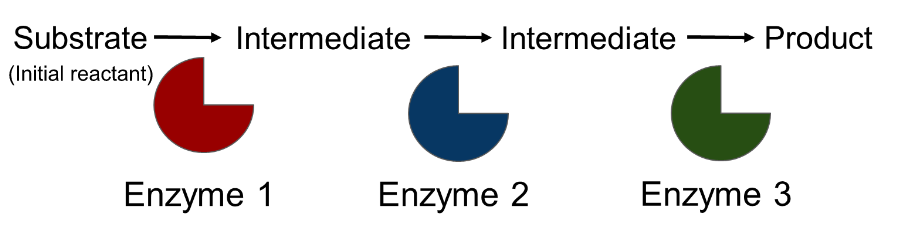
Metabolic Pathways
There are two types of metabolic pathways
| Catabolic | Anabolic |
|---|---|
| Release energy by breaking down complex molecules into simpler compounds | Consumes energy by building complex molecules from simpler compounds |
| Hydrolysis | Dehydration reaction |
Energy
○ ==Energy==: The ability to do work
- Organisms need energy to survive and function
○ ==Kinetic Energy==: Energy associated with motion
○ ==Thermal Energy==: Energy associated with the movement of atoms or molecules
○ ==Potential Energy==: Stores energy (due to location or structure)
○ ==Chemical Energy==: Potential energy available for release in a chemical reaction
- Energy can be converted from one form to another
Laws of Thermodynamics
The study of energy transformation in matter is called thermodynamics
The laws apply to the universe as a whole

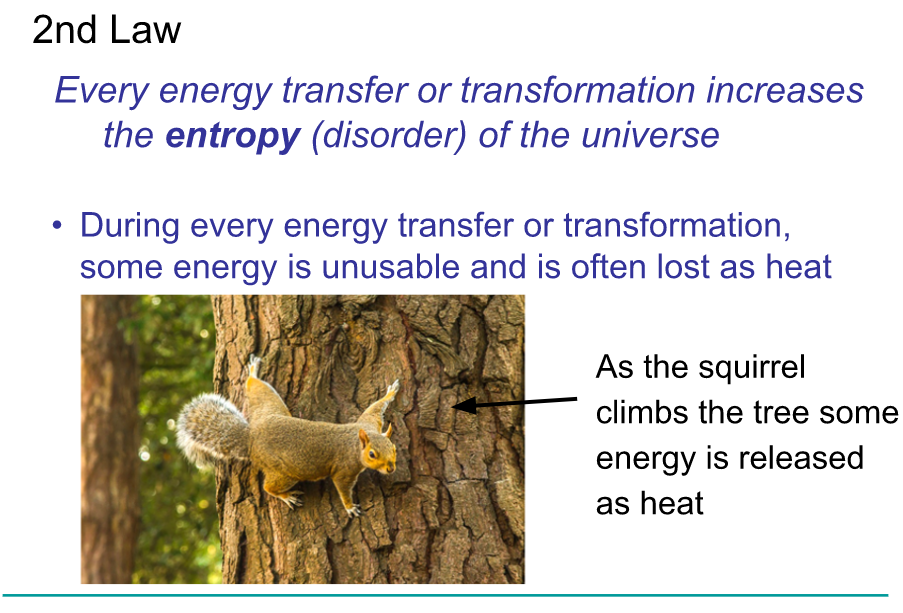
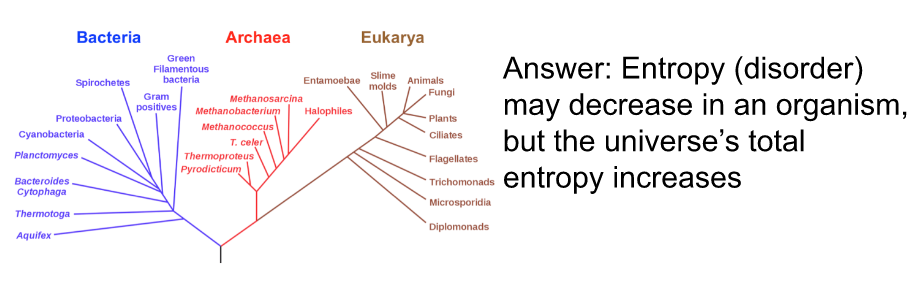
Misconception Checks
Cells create organized structures by taking in less organized storing materials (ie make proteins from amino acids). If this is true, how do cells not violate the 2nd law of thermodynamics?

In comparison to early life on Earth, organisms today are highly organized and complex. How does this increase in organization over time not violate the 2nd law of thermodynamics?
Free Energy
Since the laws of thermodynamics apply to the universe as a whole, scientists use a concept called free energy to determine the likelihood of reactions in organisms, or if the reactions are energetically favorable
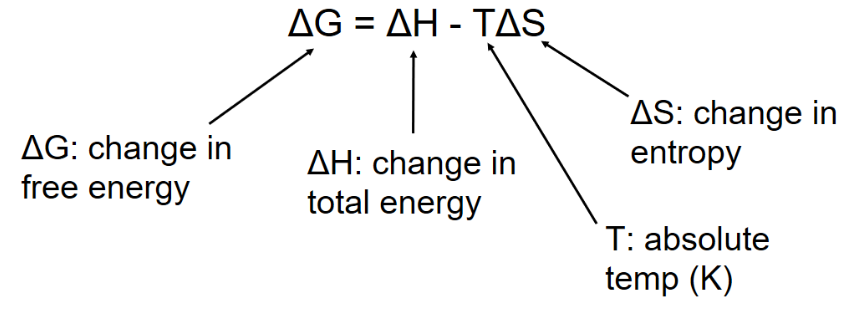 The free energy change of reactions determine whether or not the reaction occurs spontaneously
The free energy change of reactions determine whether or not the reaction occurs spontaneously
(Spontaneously refers to no outside input of energy required)
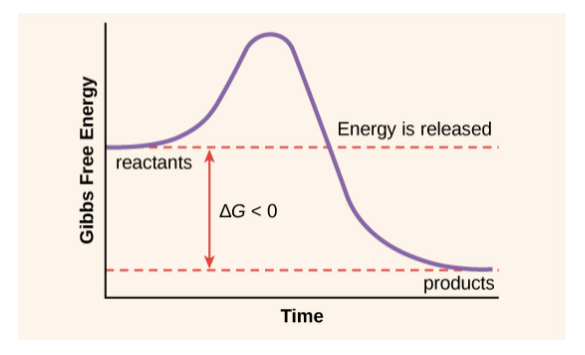
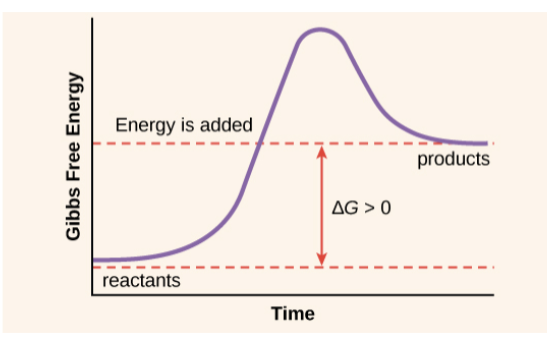
Based on free energy changes, chemical reactions can classified as exergonic or endergonic
| Exergonic Reactions | Endergonic Reactions |
|---|---|
 |  |
| Catabolic | Anabolic |
| Releases energy | Reactions absorb energy |
| Always negative | Always positive |
| Spontaneous | Not spontaneous |
| EX: Cellular respiration | EX: Photosynthesis |
- When Delta G is closer to 0, less energy is required
- When Delta G is farther from 0, more energy is required
Why some reactions occur spontaneously:
During a spontaneous change, free energy decreases and the stability of a system increases
○ ==Equilibrium==: Maximum stability of a state
- A process is spontaneous and can perform work only when it is moving toward equilibrium
- - -
Cellular work
Cells perform three kinds of work.
- ==Mechanical==: Movement (ex. Beating cilia, contracting muscles, etc.)
- ==Transport==: Actively pumping substance across membranes
- ==Chemical==: Synthesis of molecules (i.e building polymers)
To do work, cell manage energy resources by energy coupling
○ ==Energy coupling==: The use of an exergonic process to drive an endergonic process.
ATP
Most energy coupling is mediated by ATP
==ATP (Adenosine triphosphate)==: Molecule that organisms uses as a source of energy to perform work.
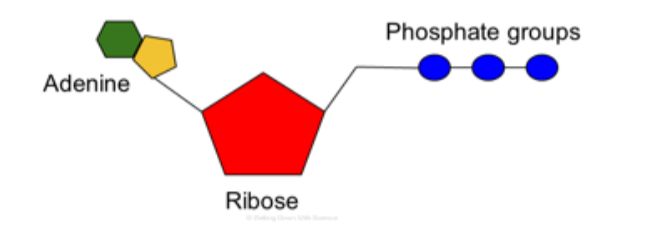 ATP couples exergonic reactions to endergonic reactions to power cellular work.
ATP couples exergonic reactions to endergonic reactions to power cellular work.
Exergonic process drive the endergonic process.
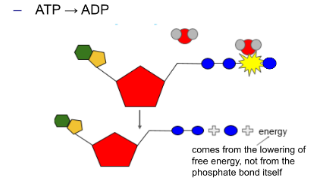
Energy is released from ATP by breaking the bond between the 2nd and 3rd phosphate in a hydrolysis reaction.
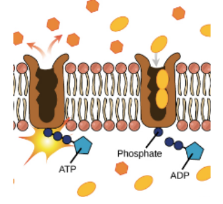 ○ ==Phosphorylation==: The released phosphate moves to another molecule to give energy.
○ ==Phosphorylation==: The released phosphate moves to another molecule to give energy.
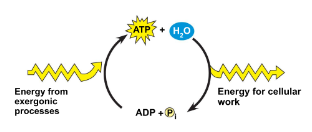
Regeneration of ATP
Rate of Metabolic Reactions:
- The laws of thermodynamics tell us if a reaction is spontaneous but it does not describe the rate of the reaction.
- Some spontaneous reactions move so slowly that it would be impossible for cells to utilize it efficiently.
Enzymes
Enzymes are types of proteins that catalyze (speeds up) reactions by lowering the activation energy. NOTE: (All enzymes are proteins)
○ ==Activation energy==: The initial energy necessary to start a chemical reaction.
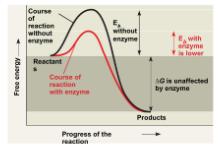
Enzyme structure
○ ==Substrate==: A molecule the enzyme acts on.
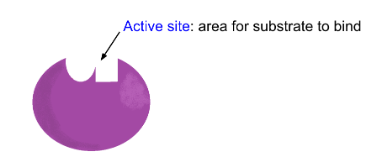 Enzyme function
Enzyme function
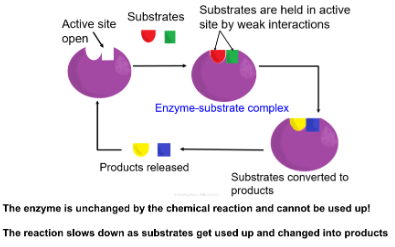
Induced fit- Enzymes will change the shape of their active site to allow the substrate to bind better. Enzymes and substrates would fit together like a lock and key. Only one enzyme for each substrate.
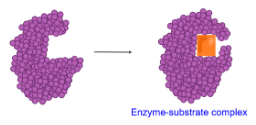 NOTE: All enzymes end with ase like lactase.
NOTE: All enzymes end with ase like lactase.
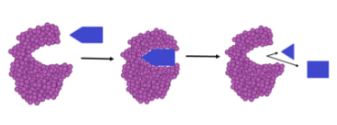
Enzyme Catabolism
Enzyme helps break down complex molecules.
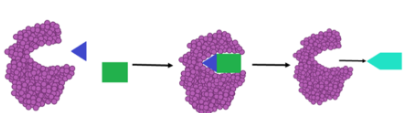
Enzyme Anabolism
Enzymes help build up complex molecules.
 Effects of Conditions on Enzyme Activity
Effects of Conditions on Enzyme Activity
- Temperature
- pH
- Chemicals
- Other environmental factors (salinity)
NOTE: Change in shape means a change in function
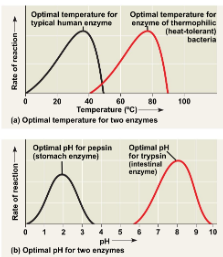 When an enzyme is no longer able to function is called ==denature==. The enzyme activity increases.
When an enzyme is no longer able to function is called ==denature==. The enzyme activity increases.
- - -
Enzyme Cofactors, Inhibitors, and Activators
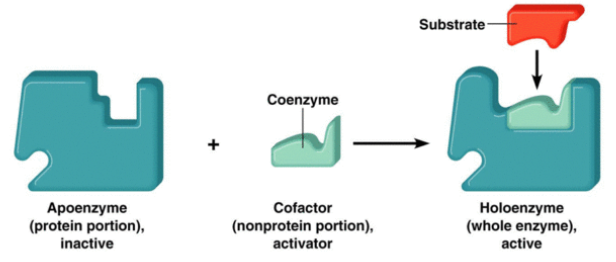
○ ==Cofactors==: Non-protein enzyme helpers
- Inorganic cofactors consists of metals
- Can be bound loosely or tightly
○ ==Holoenzyme==: Enzyme with a cofactor attached
○ ==Coenzymes==: Organic cofactors
==EX==: Vitamins
Enzyme Inhibitors
○ ==Enzyme Inhibitors==: Reduce the activity of a specific enzyme
Inhibition can be permanent or reversible
- Permanent - Inhibitors bind with covalent bonds
==EX==: Toxins and poisons
- Reversible - Inhibitor binds with weak interactions
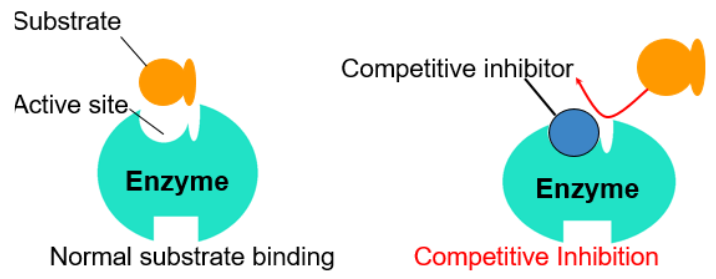
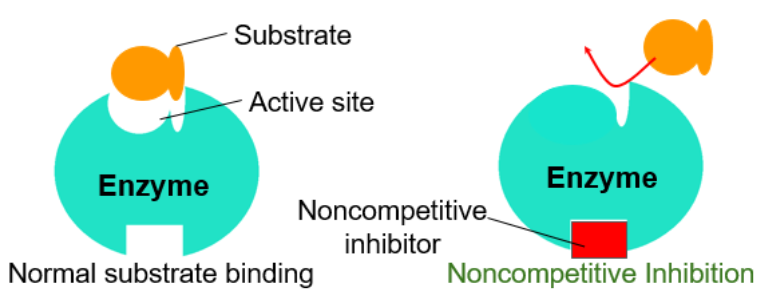
| Competitive Inhibitors | Noncompetitive Inhibitors |
|---|---|
| Binds to the active site of an enzyme, competing with the substrate | Binds to another part of the enzyme (alloesteric site), causing the enzyme to change shape and preventing the substrate from binding |
| Blocks substrate from binding | |
| Can be reversed with increased substrate concentrations | |
 |  |
Regulation of Chemical Reactions
A cell must be able to regulate its metabolic pathways
How? →
- Controls where and when enzymes are active
- Switch genes that code for enzymes on and off
Allosteric Regulation
Alloesteric enzymes have two binding sites
- One active site
- One allosteric site (regulatory site, site other than the active site)
○ ==Allosteric Regulation==: Molecules bind (noncovalent interactions) to an allosteric site which changes the shape and function of the active site
May result in inhibition (by an inhibitor) or stimulation (by an activator) of the enzymes activity
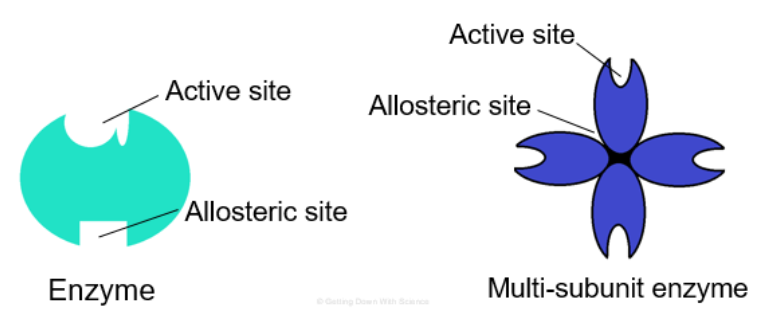
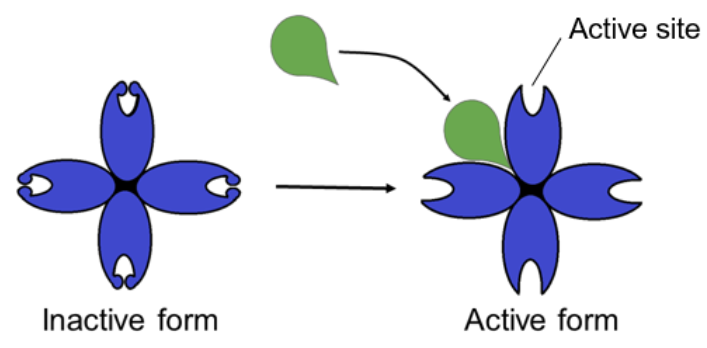
○ ==Allosteric Activator==: Substrate bind to allosteric site and stabilizes the shape of the enzyme so that the active sites remain open
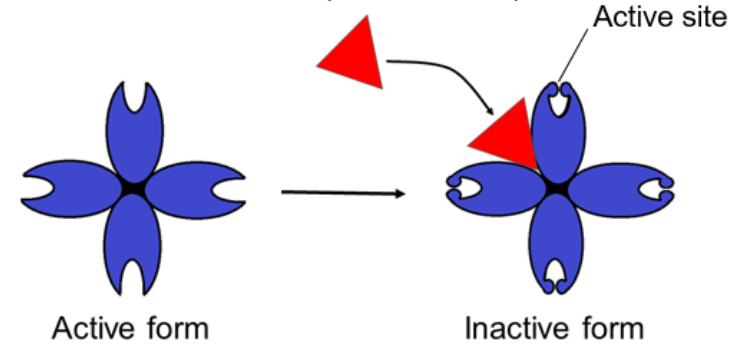
○ ==Allosteric Inhibitor==: Substrate binds to allosteric site and stabilizes the shape of the enzyme so that the active sites are closed
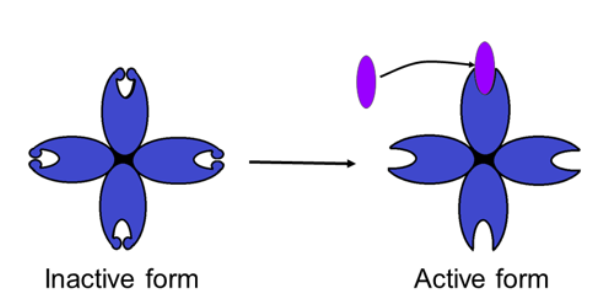
○ ==Cooperativity==: Substrate binds to an active site and stabilizes the active form (of an enzyme with many active sites)
Feedback Inhibition
The end product of the metabolic pathway shuts down the pathway
Prevents a cell from wasting chemical resources by synthesizing more product than is needed
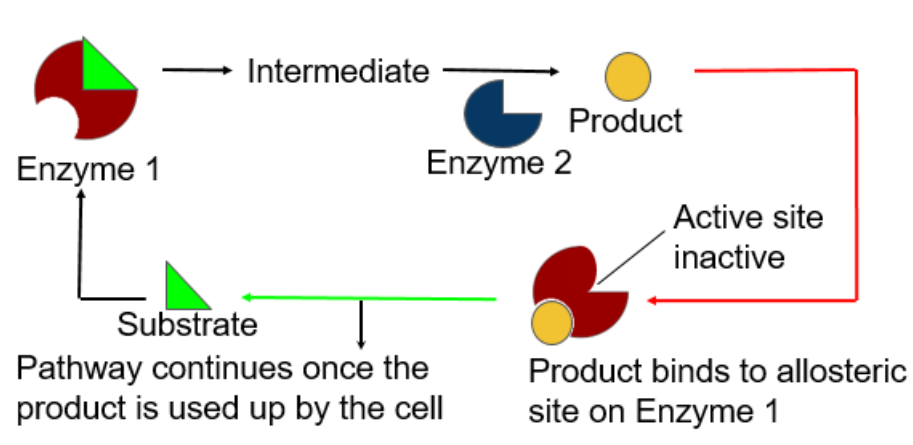
- - -
Intro to Photosynthesis
Photosynthesis
○ ==Photosynthesis==: The process that converts solar energy into chemical energy (glucose)
- Directly or indirectly, photosynthesis nourishes almost the entire world
| Autotrophs | Heterotrophs |
|---|---|
| Produces own food (organic molecules) from simple substances in their surroundings | Unable to produce their food |
| Survives off other organisms |
Why are autotrophs also called producers? →They produce their food (organic molecules)
What type of autotroph are almost all plants? →Photoautotrophs (photo = light)
How do heterotrophs obtain their organic material? →Through the organisms
How do heterotrophs depend on photoautotrophs?→ For food and oxygen
Evolution of Photosynthesis
Photosynthesis first evolved in prokaryotic organisms
○ ==Cyanobacteria==: Early prokaryotes capable of photosynthesis
- Oxygenated the atmosphere and early earth
- Prokaryotic photosynthesis pathways were the foundation for eukaryotic photosynthesis
Site of Photosynthesis
Leaves are the primary location of photosynthesis in most plants
○ ==Chloroplast==: Organelle for the location of photosynthesis
○ ==Mesophyll==: The cells that make up the interior tissue of the leaf
○ ==Stomata==: Pores in leaves that allow CO2 in and O2 out
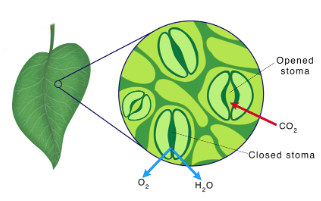
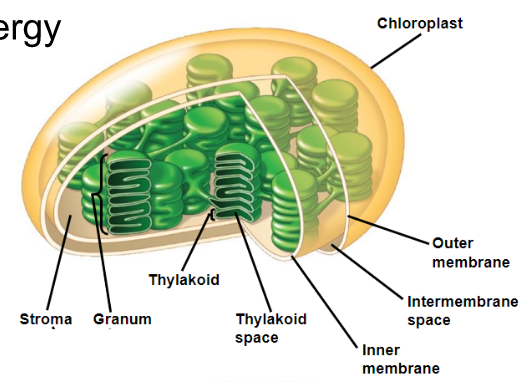
Structures of a Chloroplast
Chloroplasts are surrounded by a double membrane
○ ==Stroma==: Aqueous internal fluid
○ ==Thylakoids==: Form stacks as grana
○ ==Chlorophyll==: Green pigment in thylakoid membranes that absorbs light energy
Photosynthesis
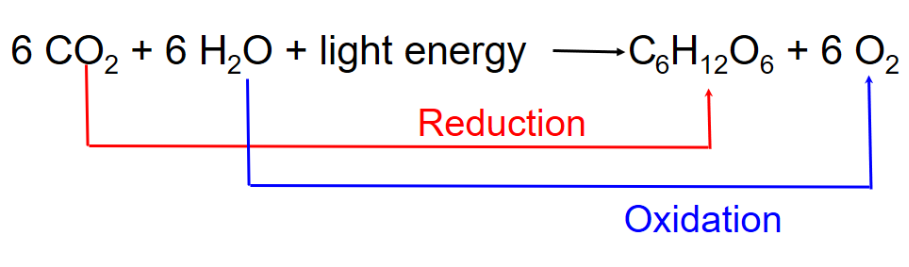
Photosynthesis can be summarized as the following equation:
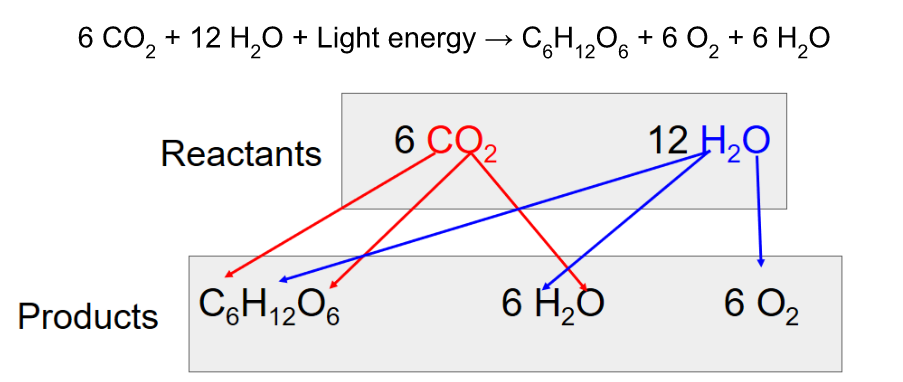
Photosynthesis splits H2O into H and O
○ ==Redox Reaction==: Reaction involving complete or partial transfer of one or more electrons from one reactant to another
- In photosynthesis: The electrons are transferred with H+ (From split H2O) to CO2, reducing it to sugar
- H2O is oxidized and CO2 is released
Remember: “OILRIG”
- Oxidation is the loss of e-
- Reduction is the gain of e-
Redox reactions play an important role in both photosynthesis and cellular respiration
Stages of Photosynthesis
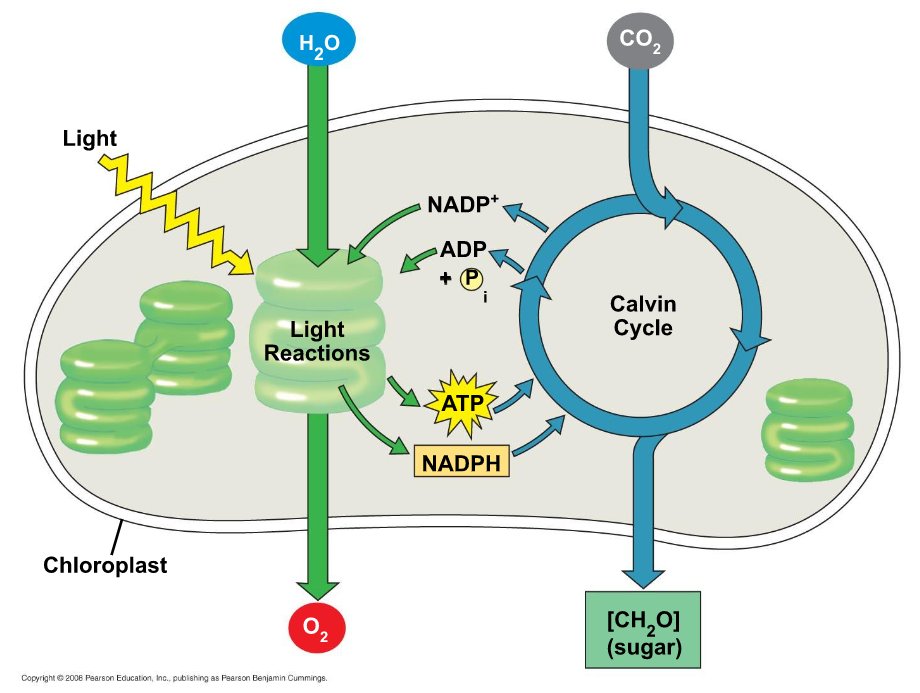
There are two stages to photosynthesis: the light reactions and the Calvin cycle
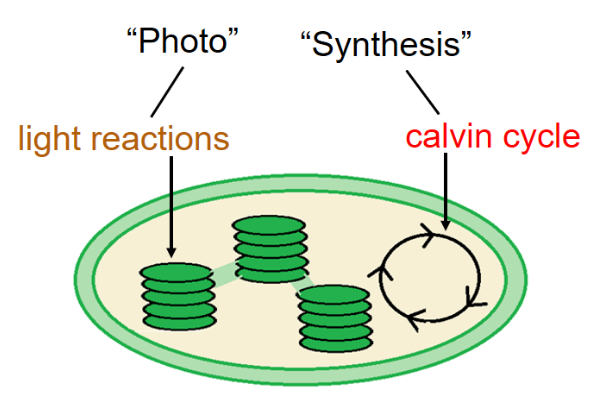
The Light Reactions (in the thylakoids)
Split H2O
Release O2
Reduce NADP+ to NADPH
Generates ATP from ADP by photophosphorylation
- The thylakoids transform light energy into the chemical energy of ATP and NADPH
The Calvin Cycle (in the stroma)
Forms sugar from CO2, using ATP and NADPH
Begins with carbon fixation, incorporating CO2 into organic molecules
Understanding Light
○ ==Light==: Electromagnetic energy
- Made up of particles of energy called photons
- Travels in waves
○ ==Wavelength==: The distance from the crest of one wave to the crest of the next
○ ==Electromagnetic Spectrum==: The entire range of waves
- Short wavelength = high energy
- Long wavelength = low energy
When light interacts with matter, it can be:
- Reflected (Color we see is reflected wavelengths)
- Transmitted
- Absorbed
What can absorb visible light? → Pigments
○ ==Absorption Spectrum==: The pigment’s absorption versus wavelength
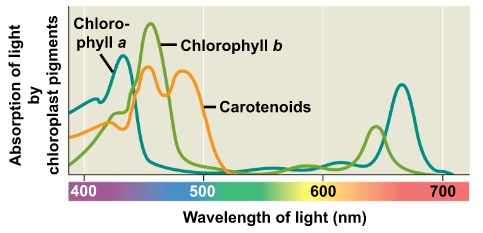
○ ==Action Spectrum==: Relative effectiveness of different wavelengths of radiation in driving a process
Photosynthetic Pigments
○ ==Chlorophyll a==: Primary pigment used in photosynthesis, used in light reactions (blue/green pigment)
○ ==Chlorophyll b==: Accessory pigment (yellow/green pigment)
○ ==Carotenoids==: Broadens the spectrum of colors that drive photosynthesis (yellow/orange pigment)
○ ==Photoprotection==: Carotenoids absorb and dissipate excess light energy that could damage chlorophyll or interact with oxygen
- - -
Light reactions occur in the thylakoid membrane in photosynthesis. Photosynthesis converts solar energy to chemical energy.
Chemical energy can be in two forms:
- NADPH
- ATP
The cell accomplishes this conversion by using light energy (photons) to excite electrons.
Chlorophyll absorbs a photon of light and the electrons (e⁻) are boosted from a ground state to an excited state
- e⁻ is unstable
- Falls back to ground state
As the electrons fall back to the ground state they release:
- Energy as heat
- Emit photons as fluorescence
NOTE:
- e⁻ means electrons
- H⁺ means protons
Photosystems:
A photosystem is a reaction-center complex surrounded by light-harvesting complexes.
○ ==Reaction center==: A complex of proteins associated with chlorophyll a and an electron acceptor
○ ==Light-harvesting complex==: Pigments associated with proteins
- Funnel energy of photons to the reaction center.
Primary electron acceptor: Accepts the excited electrons from chlorophyll a.
Two photosystems in the thylakoid membrane
- Photosystem II (2):
- Reaction center P680
- Absorbs light
- Functions first
- Photosystem I (1):
- Reaction center P700
- Absorbs light at 700nm
○ ==Linear electron flow==: The primary pathway that photosynthesis occurs in because it produces ATP and NADPH using light energy.
Inside photosystem II:
Light energy (photon) causes an e⁻ to go from an excited state back to a ground state. This process repeats itself and stops when it reaches P680. the excited electron gets transferred to the primary electron acceptor.
- H2O splits into 2 e⁻ (Used to reduce P680)
- 2 H⁺ (Reduced into thylakoid space)
- 1 oxygen atom (Immediately bonds to another oxygen atom)
Linear electron flow: Each excited electron will fall down an electron transport chain from PS II to PSI.
- Energy is generated by creating a proton gradient.
- ATP synthesis is driven by the diffusion of H⁺ (protons) across the membrane.
Inside photosystem I:
- Transferred light energy excites P700, which loses an electron to an electron acceptor.
- P700 accepts an electron passed down from PS II via the electron transport chain.
- Each electron “falls down an electron transport chai from the primary electron acceptor of PS I to the protein ferredoxin (Fd).
- The electrons are transferred to NADP⁺ and reduced to NADPH.
- The electrons of NADPH are available for the reactions of the Calvin cycle.
| INPUTS | OUTPUTS |
|---|---|
| H2O | O2 |
| ADP | ATP |
| NADP⁺ | NADPH |
Light reaction summary
- Converts solar energy to chemical energy.
- Chemical energy is in two forms: NADPH and ATP
- Water is split
- Provides a source of electrons and protons (H+)
- Releases O2 as a by-product
- Light absorbed by chlorophyll drives the transfer of electrons and hydrogen ions from H2O to an electron acceptor called NADP⁺
- NADP⁺ is reduced to NADPH
- Generates ATP by phosphorylating ADP
Cyclic Electron flow
- Uses only photosystem I and produces ATP, but not NADPH
- Generates surplus ATP, satisfying the higher demand in the Calvin cycle
- Some organisms have PS I but not PS II
- Cyclic electron flow is thought to have evolved before linear electron flow
- Cyclic electron flow may protect cells from light-induced damage
- - -
Calvin Cycle and Plant Adaptations
- The Calvin Cycle is a cyclic electron flow
- Occurs in the stroma of the chloroplast
- Uses ATP and NADPH to reduce CO2 to sugar (G3P)
- For the net synthesis of 1 G3P molecule, the cycle must take place 3 times
3 Phases of the Calvin Cycle
==Phase 1==: Carbon Fixation
- CO2 is incorporated into the Calvin Cycle 1 at a time
- Each CO2 attaches to a molecule of RuBP (Catalyzed by the enzyme RuBisCo)
- Forms 3-phosphoglycerate
==Phase 2==: Reduction
- Each molecule of 3-phosphoglycerated is phosphorylated by ATP (uses 6 total)
- Becomes 1,3-bisphosphoglycerate
- 6 NADPH molecules donate electrons to 1,3-biphosphoglycerate
- Reduces to G3P
- 6 molecules of G3P are formed, but 1 is counted as a net gain (the other 5 G3P molecules are used to regenerate RuBP)
==Phase 3==: Regeneration of RuBP
- 5 G3P molecules are used to regenerate 3 molecules of RuBP
- Uses 3 ATP for regeneration
- The cycle is now ready to take in CO2 again
| Inputs: | Outputs: |
|---|---|
| 3 CO2 | 1 G3P |
| 9 ATP | 9 ADP |
| 6 NADPH | 6 NADP+ |
Calvin Cycle Summary
- Uses: NADPH, ATP, and CO2
- Produces: A 9 3-C sugar G3P
- Phases:
- Carbon fixation
- Reduction
- Regeneration of RuBP
The problem for C3 Plants
- C3 Plants: Most plants
- Photorespiration:
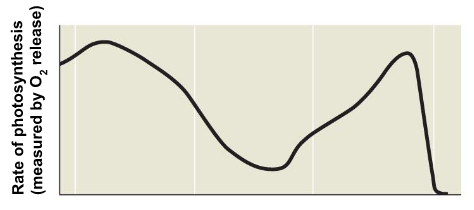
When does it Occur? → Occurs on very hot days
What do plants do in response to the heat? → Close their stomata to stop water from leaving
What impact does this have on the rate of photosynthesis? →Limits photosynthesis
Which gasses become less common in the plant? More common? → Causes less CO2 present and more O2
What does RuBisCo bind to? → O2 and uses ATP
What does the process produce? → CO2
What does the process not produce? → No sugar is produced
BAD for the plant!!!
Adaptations
Plants that live in hot, dry climates have evolved to have alternative mechanisms of carbon fixation
C4 Plants:
- Stomata partially close to conserve water
- Uses PEP Carboxylase to fix CO2 (instead of RuBisCo)
- Mesphyll cells fix CO2 into a 4-C molecules
- What happens to these 4-C molecules? → Transferred to bundle-sheath cells
- What do they release that is used in the Calvin cycle? → Releases CO2
==EX==: Corn, grasses, and sugarcane
CAM Plants:
- Open stomata at night and close during the day
- CO2 is incorporated into organic acids and stored in vacuoles
- During the day, light reactions occur and CO2 is released from the organic acids and incorporated into the Calvin cycle
==EX==: Pineapple, cacti, succulents, and jade
- - -
Energy cycling and harvesting
Living cells require chemical energy for metabolism.
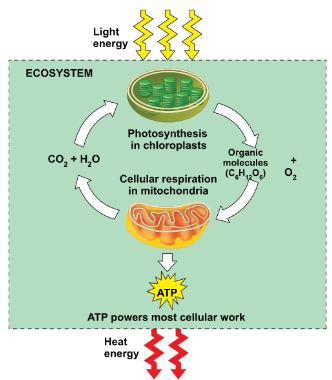
○ ==Cellular respiration==: Process in which cells harvest chemical energy stored in organic molecules (glucose) and use it to generate ATP.
- NOTE:
- Autotrophs make their own glucose through the process of photosynthesis.
Heterotrophs get their glucose from consuming other organisms.Photosynthesis equation: 6CO2 + 6H2O----------C6 H12 O6 +6O2
Cellular respiration: C6 H12 O6 +6O2-----------------6CO2 + 6H2O + ATP energy
NOTE: They’re the same formula, just reversed.
Cellular respiration:
Catabolic breakdown of glucose:
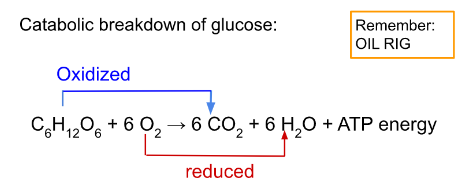
The oxidation of glucose transfers e⁻ to a lower energy state, releasing energy to be used in ATP synthase.
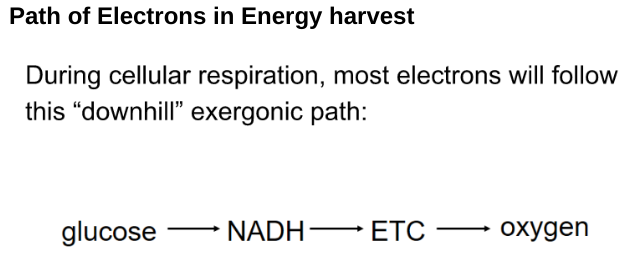
Path of Electrons in Energy harvest
Energy Harvesting
Glucose is broken down in steps to harvest energy
- Electrons are taken from glucose at different steps
- Each e⁻ taken travels with a proton
- Dehydrognases take 2e⁻ and 2 protons glucose
- It is known as an oxidizing agent
- Transfers 2 e⁻ and 1 proton to the coenzyme NAD⁺. Reduces to NADH (stored the energy)
- The other protons are released into the surrounding solution as H⁺.
- NADH carries e⁻ to the electron transport chain.
- Electron transport chain (ETC): A sequence of membrane proteins that shuttle electrons down a series of redox reactions.
- Releases energy uses to make ATP
ETC transfers e⁻ to O2 (the final electron acceptor) to make H2O.ntro to Cellular Respiration
Location: Mitochondria
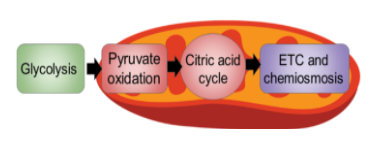
Stages of Cellular Respiration
There are 3 stages of cellular respiration
Glycolysis
Pyruvate oxidation and the citric acid cycle
Oxidative phosphorylation (ETC and chemiosmosis)
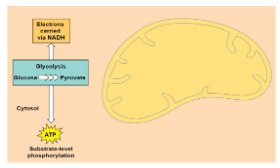
Glycolysis
Starting point of Cellular respiration.
Occurs in the cytosol
Splits glucose (6C) into 2 pyruvates (3C)
Glycolysis model:
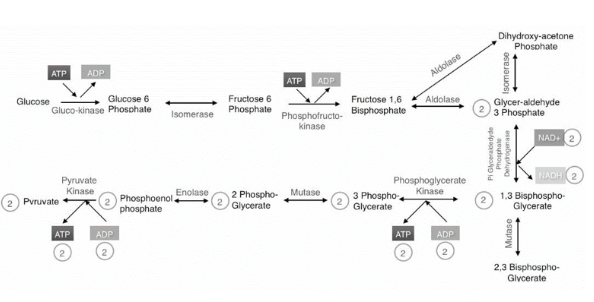
Summarized Pathway:
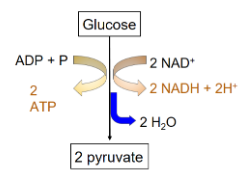 Two stages:
Two stages:
Energy investment stage: The cell useless ATP to phosphorylate compounds of glucose. Energy payoff stage: Energy is produced by substrate-level phosphorylation. Net energy per 1 glucose: 2 ATP 2 NADH
Glycolysis Summary
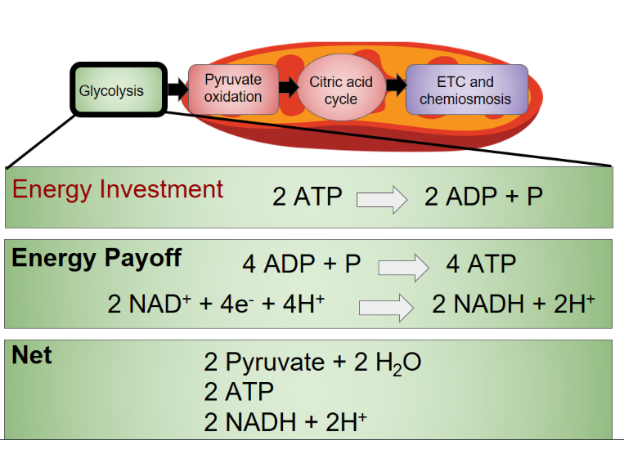
- - -
Aerobic Cellular Respiration
Glycolysis Recap
- The starting point of cellular respiration
- Occurs in the cytosol
- Splits glucose
Products: → 2 Pyruvate, 2 ATP, 2 NADH
2 ATP and 2 NADH are by-products. Pyruvates are the main products
Pyruvate Oxidation and Citric Acid Cycle
If oxygen is present, the pyruvate enters the mitochondria (eukaryotic cells)
○ ==Aerobic==: Requires oxygen
Which stages are aerobic? → The steps that occur in the mitochondria
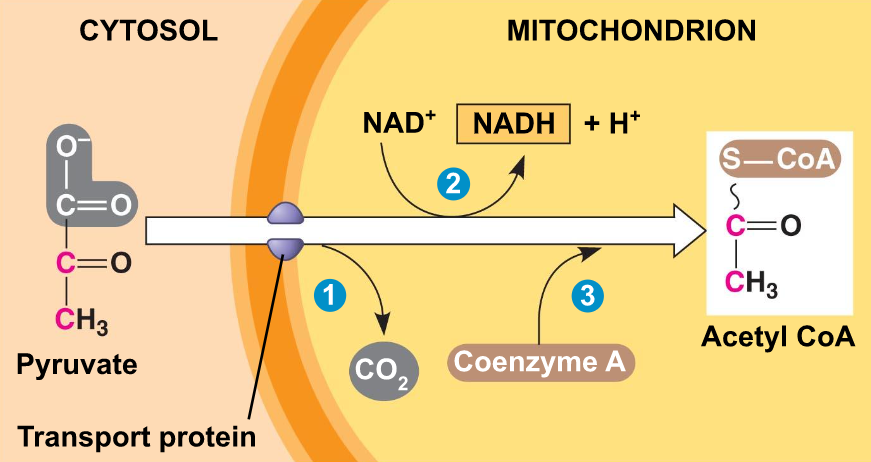
Pyruvate is oxidized into acetyl coA
What is acetyl coA used to make? → Citrate in the citric acid cycle
What other molecules are produced? → 2CO2 and 2NADH
Pyruvate Oxidation
Citric Acid Cycle
- Also known as the Krebs Cycle
- Occurs in the mitochondrial matrix
- Turns acetyl coA into citrate
- Releases CO2
- ATP synthesized
- Electrons transferred to NADH and FADH+
How many steps is the Citric Acid Cycle? → 8 steps
The acetyl group of acetyl coA joins the cycle by combining with oxaloacetate, forming citrate
What happens in the next 7 steps? → Decompose the citrate back to oxaloacetate, forming a cycle
Where do the electrons from the NADH and FADH2 go? → They go to the electron transport chain (ETC)
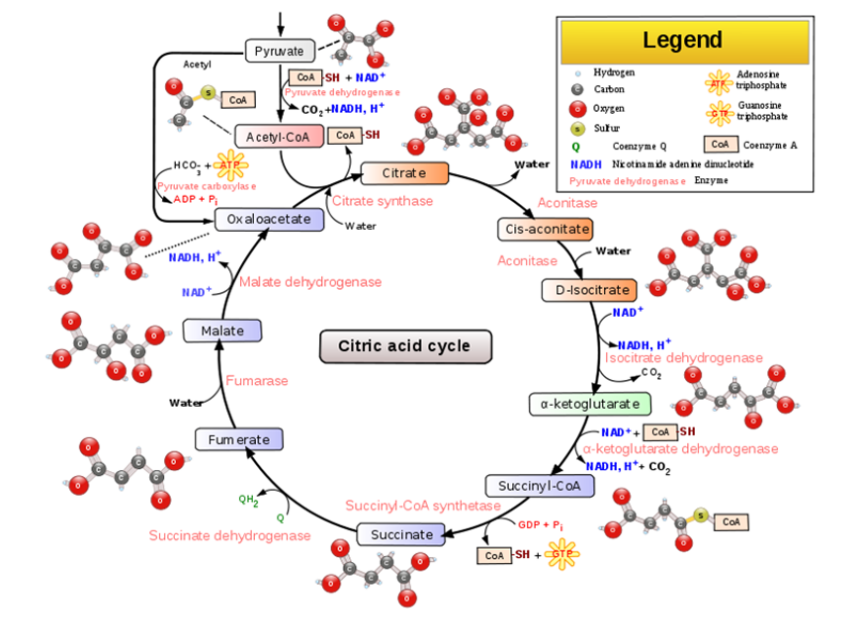
Summarized pathway (per glucose molecule):
| Inputs: | Outputs: |
|---|---|
| 2 acetyl coA | 2 ATP |
| 6 NADH | |
| 4 CO2 | |
| 2FADH2 |
Oxidative Phosphorylation
Oxidative Phosphorylation consists of:
- Electron transport chain
- Chemiosmosis
Electron Transport Chain (ETC)
- Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food
- Electrons are transferred from NADH or FADH2 to the ETC
- These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation
- The ETC is located in the inner membrane of the mitochondria
- Collection of proteins
- As the electrons “fall”, proteins alternate between reduced (accepts e-) and oxidized (donates e-) state
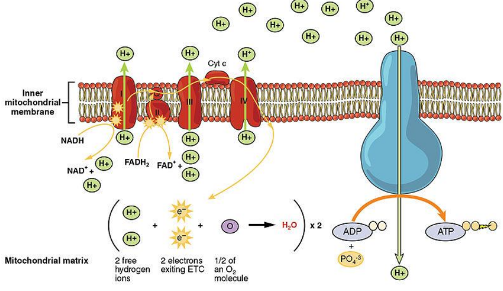
- The Cristae (folds) increase the surface area for the reactions to occur
- Does not produce ATP directly
How does it help manage the release of energy? → Creates several small steps for the “fall” of electrons
- The final electron acceptor is oxygen
What molecule forms? → H2O→ Each oxygen pairs with 2 H+ and 2 e-
- One major function of the ETC is to create a proton (H+) gradient across the membrane
- As proteins shuttle electrons across the ETC, they also pump H+ into the intermembrane space
What does this gradient power? → Chemiosmosis
Chemiosmosis
○ ==ATP Synthase==: The enzyme that makes ATP
Where does the energy for this come from? → Uses energy from the H+ proton gradient
- H+ ions flow down their gradient through ATP synthase
- It acts like a rotor
How does it activate catalytic sites to turn ADP + P into ATP? → When H+ binds, the rotor spins
- Produces about 26-28 ATP per glucose
Summary Chart
| Summary: | Inputs: | Outputs: |
|---|---|---|
| Glycolysis | - 1 glucose | - 2 Pyruvate- 2 ATP- 2 NADH |
| Pyruvate Oxidation | - 2 pyruvate | - 2 acetyl coA- 2 CO2- 2 NADH |
| Citric Acid Cycle | - 2 acetyl coA | - 4 CO2- 2 ATP- 6 NADH- 2 FADH2 |
| Oxidative Phosphorylation | - 10 NADH- 2FADH2 | - 26-28 ATP |
| Total ATP: | 30-32 |
- - -
NOTICE: This note sheet will no longer be active. Please see Google Doc for up-to-date notes:
https://docs.google.com/document/d/10yO4SiIAARKozGcpN1R684ft2XLvnbN3KF4_wwryjkw/edit