Topic 7 Regents Chemistry Review: Solutions & Solubility
Topic Overview
Most of the materials that you use every day are not pure substances. It is more likely that they are mixtures. This topic will explore an important type of mixture, the solution. The nature and properties of solutions are important concepts used in chemistry. One reason they are so important is that most chemical reactions take place in solutions. In this topic you will study the nature and properties of solutions and ways to express the concentration of solutions.
Solutions
A solution is a homogenous mixture of substances in the same physical state. Solution contain atoms, ions, or molecules of one substance spread uniformly throughout a second substance. When salt (NaCl) is stirred into water, the individual ions of the salt separate and uniformly spread throughout the water, forming a solution.
Types of Solutions
A solid may be dissolved in another solid. Brass is a mixture of zinc and copper. When metals are mixed to form a solution, the result is called an alloy. Air is an example of a mixture of gases forming a solution. Although solutions exists in all three states, the discussion in this topic will be limited to liquid solutions. Perhaps the most common type of solution is one in which a solid or liquid is dissolved in a liquid.
The terms solute and solvent are commonly used to identify the parts of a solution. In general terms, the solute is the substance that is being dissolved, and it is the substance present in the smaller amount. When solid sodium nitrate (NaNO3) dissolves in water, the sodium nitrate is the solute. The substance that dissolves the solute is the solvent, and is present in the greater amount. Water is, perhaps, the most common solvent. Water solutions are called aqueous solutions, and the notation (aq) is used in equations to show that the substance has been dissolved in water. Once the salt and the water are stirred and the mixture becomes homogenous, the dissolved particles will not settle. Liquid solutions are clear and light will pass through the solution without being dispersed.
Solutions may or may not have color. For example, solutions of copper salts have a characteristic blue color, while a solution of sodium nitrate is colorless.
The following list summarizes characteristics of liquid solutions:
Solutions are homogenous mixtures
Solutions are clear and do not disperse light
Solutions can have color
Solutions will not settle on standing
Solutions will pass through a filter
Solubility Factors
You've noticed that some things easily dissolve in water or other solvents. When you make a cup of coffee, certain materials in the coffee grounds dissolve but other materials don't. Sugar will readily dissolve in the cup of coffee but the spoon you use to stir the solution does not dissolve. How much of a solute will dissolve in a certain amount of solvent at a certain temperature is known as solubility. Materials with a high solubility are said to be soluble; materials with a low solubility are said to be insoluble. What factors determine the solubility of a solute in a solvent?
Nature of Solute and Solvent When sodium chloride dissolves in water it does so because its positively and negatively charged ions are attracted to the oppositely charged ends of the polar water molecule. The positively charged sodium ions are attracted to the negative pole of the water molecules. In like manner, the negatively charged chloride ions are attracted to the positive end of the water dipole and are dissolved. The attractive forces between the ions and water molecules are called ion-dipole forces, and are greater than the forces of attraction between the ions themselves. Ionic and polar substances dissolve in polar solvents
Nonpolar substances, such as fats, do not dissolve in water because there aren't strong attractive forces between the fat molecules and the water molecules. Fat molecules will dissolve in nonpolar solvents. The forces that hold the nonpolar molecules to each other are quite weak, and the molecules simply mix together. The term "like dissolves like” is often used to describe what solutes will dissolve in what solvents.
Temperature As temperature increases, most solids become more soluble in water. A few exceptions exist. Gases react in the opposite manner. As temperature rises, the solubility of all gases in liquids decreases.
Pressure Pressure has little or no effect on the solubility of solid or liquid solutes. Pressure does not affect the solubility of gases in liquids. As pressure increases, the solubility of the gases in liquids increases. When a can of soda is opened, the pressure decreases. The carbon dioxide is no longer soluble at lowered pressure, and it escapes as bubbles.
Solubility Curves
Solubility info may be presented in different ways. Solubility curves in the Reference Table for Physical Setting/Chemistry presents quantitative information showing the relationships of grams of solute that may be dissolved at various temperatures. Solubility Guidelines provides some general guidelines about the solubility of ionic substances.
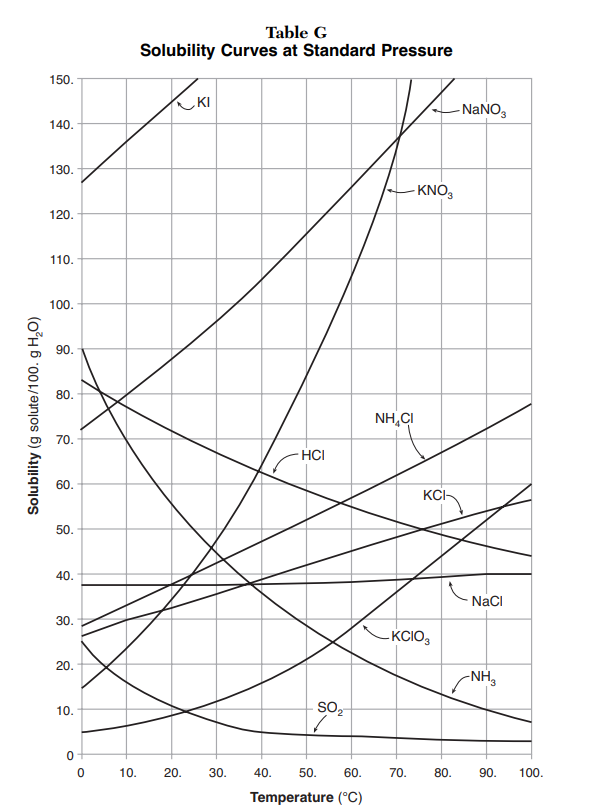
Solubility Graphs The Solubility Curves table shows the number of grams of a substance that can be dissolved in 100 grams of water at temperatures between 0°C and 100°C. Each line represents the maximum amount of that substance that can be dissolved at a given temperature. All of the lines that show an increase in solubility as temperatures increase represent solids being dissolved in water. Although these lines on the graph show an increase, a few solids, such as cesium sulfate (Cs2SO4), become less soluble as temperature increases.
Three lines show decreasing solubility with increasing temperature. These three lines represent the gases NH3, HCl, and SO2. The solubility of all gases decrease with increasing temperature.
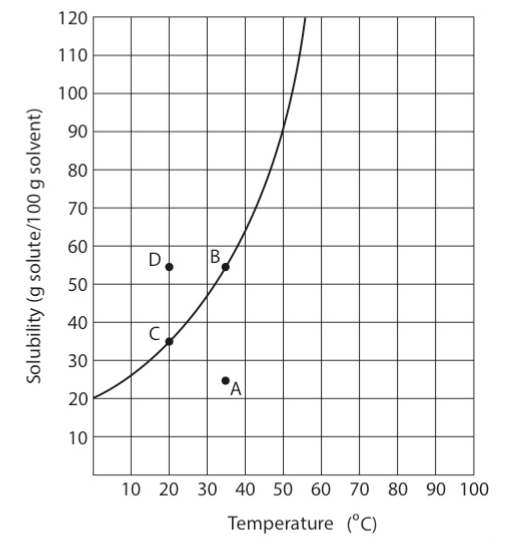
This graph shows four positions relative to a line of maximum solubility. Position A is below the maximum line of solubility. At this position, the temperature is 35°C, and 25 grams of solute X dissolve. Because at this point the solution holds less solute than the maximum it can hold, the solution is said to be unsaturated. If there is not a temperature change, an additional 30 grams can be added to X to position B.
Position B is on the line of maximum solubility. A solution that contains a maximum amount of solute that will dissolve at a specific temperature is saturated. At this position, the solution contains 55 grams of solute X. The addition of more solid solute will not result in more being dissolved. Any additional solid that is added will simply settle to the bottom of the container.
If the temperature is reduced to 20°C, only 35 grams of X can dissolve. When the temperature of the solution at Position B is reduced, the most likely event is that the excess 20 grams of solute X will precipitate, and the solution will remain saturated at point C. On rather rare occasions, as the temperature decreases, crystals do not form and the substance may be at position D. At position D, there is more solid dissolved than normal. A solution that holds more solute than is present in a saturated solution at that temperature is supersaturated. These solutions are quite unstable. The addition of a single solid crystal of the substance will cause additional solid to form, and the solution will return to a saturated condition. If no temperature change occurred, the solution would become saturated at point C, with 20 g of the substance precipitating. The only way to make a supersaturated solution is to cool a saturated solution in which there are no crystals or impurities, such as dust, present.
Solubility Guidelines for Aqueous Solutions
The Solubility Guidelines of Reference Tables for Physical Setting/Chemistry contains some guidelines for the solubility of common ionic compounds. The table shows that all compounds of the ammonium and the nitrate ion are soluble. All of the halide ions, such as CI, form compounds that are soluble, but three exceptions are listed. Silver chloride is not soluble, nor are Pb2+ nor Hg22+ chlorides, and they are precipitates if they form in a double-replacement reaction. This table is useful in predicting whether or not a precipitate will form when two ionic solutions are mixed. A reaction will take place if one or both of the products is listed as insoluble.
Recognizing Unsaturated, Saturated, and Supersaturated Solutions Because solutions are clear, it is difficult to simply look at a solution and determine whether it is unsaturated, saturated, or supersaturated. One method of recognizing the type of solution narrows the choices. If a solution contains some undissolved solute, it must be a saturated solution.
The addition of a solute crystal can also be used to determine its condition. If it dissolves, the original solution was unsaturated. If it simply falls to the bottom, the solution is saturated. If it causes additional crystals to form, the original solution was supersaturated.
Concentration of Solutions
Because solutions are homogeneous mixtures, their compositions can vary. Sometimes, it is adequate to refer to a solution as dilute or concentrated. However, dilute and concentrated are relative terms and are not precise regarding the amount of solute involved. In most cases it is the specific amount, or concentration, of the solute that is important. In this section you will learn several methods of expressing the specific concentration of solute in a solution.
Molarity
One of the most important methods of stating the concentration of a solution is in terms of the number of moles of solute in a given volume of solution. The molarity (M) of a solution is the number of moles of solute in 1 L of solution. The relationship is listed in Important Formulas and Equations of Reference Tables for Physical Setting/Chemistry.
Parts Per Million
Parts per million is similar to percent composition because it compares masses. Parts per million (ppm) is a ratio between the mass of a solute and the total mass of the solution. This method of reporting concentrations is useful for extremely dilute solutions when molarity and percent mass would be difficult to interpret. For example, chlorine is used as a disinfectant in swimming pools. Only about 2 g of chlorine per 1,000,000 g of swimming pool water is necessary to keep the pool sanitized. Finding molarity and percent mass would result in numbers too small to be useful. Parts per million is often used to report a measured amount of air or water pollutants.
Percent composition uses the amount present per hundred parts because it is a percent. The only difference in finding ppm is that you multiply by 1,000,000 ppm instead of 100 percent.

Colligative Properties
The freezing and boiling points of water change when nonvolatile solutes are added. When any salt is added to water, the freezing point of the water decreases. This helps explain why salt is applied to roads and sidewalks when they are covered with snow and ice. The added salt lowers the freezing point and helps melt the snow or ice. The amount of the lowering of freezing point is not only dependent on the nature of the added particle but only on the total number of dissolved particles. One mole of any particles will have the same effect on the freezing point. One mole of particles lowers the freezing point of 1000 grams of water 1.86°C.
Vapor Pressure
The molecules in a liquid are held together by rather weak forces. Polar molecules called dipoles are held in the liquid phase by dipole-dipole forces. In molecules containing hydrogen and one atom of oxygen, nitrogen, or fluorine, the force of attraction holding them in the liquid phase are hydrogen bonds.
In any sample of a liquid, some of the particles at the surface have sufficient energy to escape from their neighboring molecules and enter the gas phase. When a substance that is normally a solid or a liquid at room temperature enters the gas phase it is called a vapor. Thus, you will often hear about water vapor or gasoline vapor, as water and gasoline are normally liquids at room temperature.
As the temperature of a liquid increases, the particles have more energy, and more particles escape from the surface. These vapor particles are gaseous particles and exert pressure in the gaseous phase. The pressure that a vapor exerts is called vapor pressure. Table H of Reference Tables for Physical Setting/Chemistry is a graph showing the vapor pressure of four substances measured in pressure units of kilopascals (kPa).
Of the substances shown on the graph, propanone exerts the most pressure, about 93 kPa at a temperature of 50°C. It can be inferred that propanone has the weakest intermolecular forces holding it in the liquid phase, while ethanoic acid has the greatest, exerting only about 8 kPa of pressure.
Boiling Point
As the temperature of a liquid rises, vapor pressure increases. Finally the vapor pressure becomes equal to atmospheric pressure. At this point the gas may vaporize, not only on the surface but at any point in the container. A bubble of vapor below the surface has enough pressure that it does not collapse from the atmospheric pressure pushing against it. When a bubble can occur at any point in the liquid, the process is called boiling. The normal boiling point of a liquid is the temperature at which the vapor pressure of the liquid is 101.3 kPa, standard atmospheric pressure. Equivalent pressures are 1 atm, 760 mm Hg, and 760 torr. The heat required to change 1 mol of a substance from a liquid at its boiling point to 1 mol of a vapor is termed the heat of vaporization.
The normal boiling point of water is 100.°C. At this temperature, the vapor pressure of water is 101.3 kPa. The line representing 101.3 kPa on Table H shows the normal boiling point of ethanol to be 78°C. When the pressure is less than 101.3 kPa, the boiling point will be less than the normal value. Water will boil at about 70°C when the pressure is about 30 kPa. If the pressure is greater than normal, liquids will boil at temperatures above their normal boiling points. When atmospheric pressure is about 145 kPa, water boils at 110.°C.
Vocabulary
Boiling Point - The temperature at which a liquid changes to a vapor.
Ion-Dipole Forces - Attractive forces between an ion and a polar molecule.
Molarity - The number of moles of solute per liter of solution.
Parts Per Million (PPM) - A measurement of the concentration of a substance in a solution, defined as the number of parts of solute per one million parts of solvent.
Percent By Volume - The ratio of the volume of the solute to the volume of the solution, multiplied by 100%.
Percent Mass - The ratio of the mass of the solute to the mass of the solution, multiplied by 100%.
Saturated - A solution that contains the maximum amount of dissolved solute at a given temperature.
Solute - A substance that is dissolved in a solvent to form a solution.
Solution - A homogeneous mixture composed of two or more substances.
Solvent - The substance in which the solute is dissolved to form a solution.
Supersaturated - A solution that contains more dissolved solute than is present in a saturated solution at the same temperature.
Unsaturated - A solution that contains less solute than the maximum amount that can be dissolved at a given temperature.
Vapor - The gaseous phase of a substance that is normally liquid or solid at room temperature.
Vapor Pressure - The pressure exerted by a vapor in equilibrium with its liquid or solid phase.
