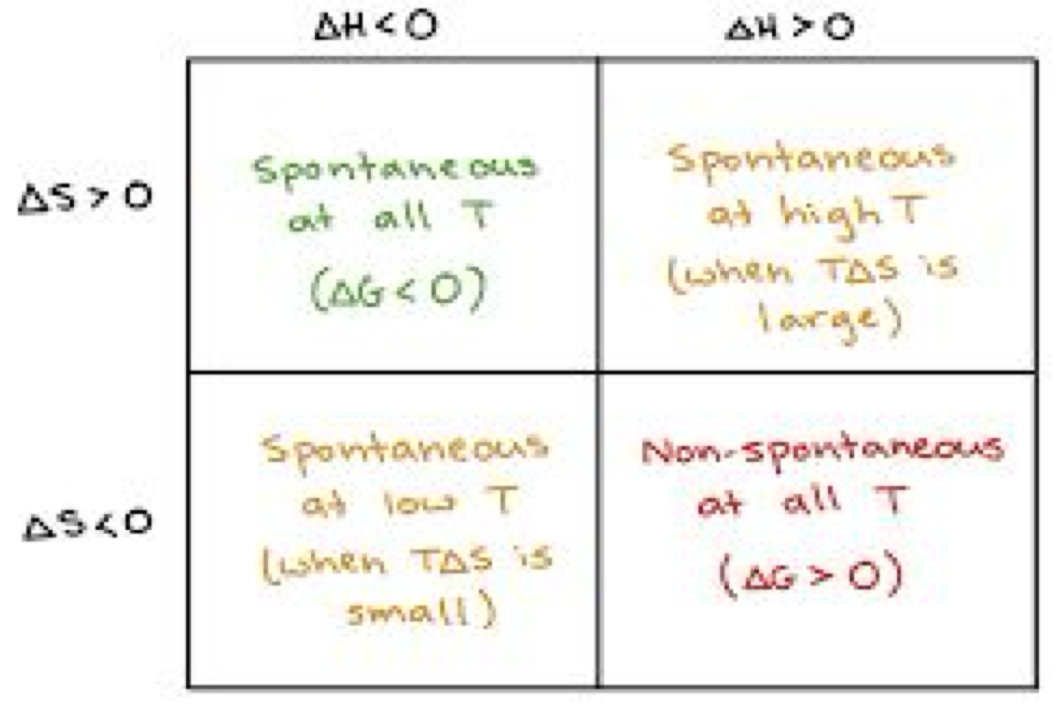
Gibbs Free Energy Notes
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
What is the difference between potential and kinetic energy?
Potential energy: the energy an object has due to its position, shape or inherent state
Kinetic energy: the energy an object possesses by virtue of its motion
Thermodynamics
A branch of physics concerned with heat and temperature and their relation to energy and work
1st Law of Thermodynamics
States that heat is a form of energy, and thermodynamic processes are therefore subject to the principle of conservation of energy; means that heat energy cannot be created or destroyed; however, it can be transferred from one location to another and converted to and from other forms of energy
2nd law of thermodynamics
As energy is transferred or transformed, more and more of it is wasted; also states that there is a natural tendency of any isolated system to degenerate into a more disordered state
What is free energy?
Thermodynamic quantity equivalent to the capacity of a system to do work
Fill in the diagram.