Lecture 2 - Cell Biology; PM, Diffusion, Osmosis
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Cell Composition
Cells are made of mostly water, organic molecules, and inorganic ions
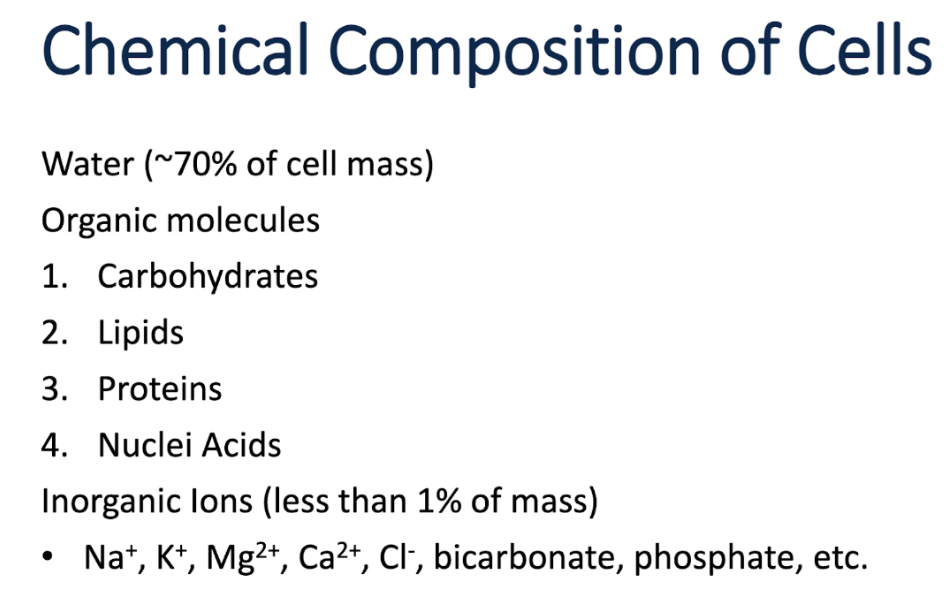
Basic cell structures
Plasma membrane functions
Helps maintain composition of intra- and extracellular fluids
Controls inward and outward traffic
Forms a framework for protein components of cell
Detects chemical messengers at cell surface
Links adjacent cells together
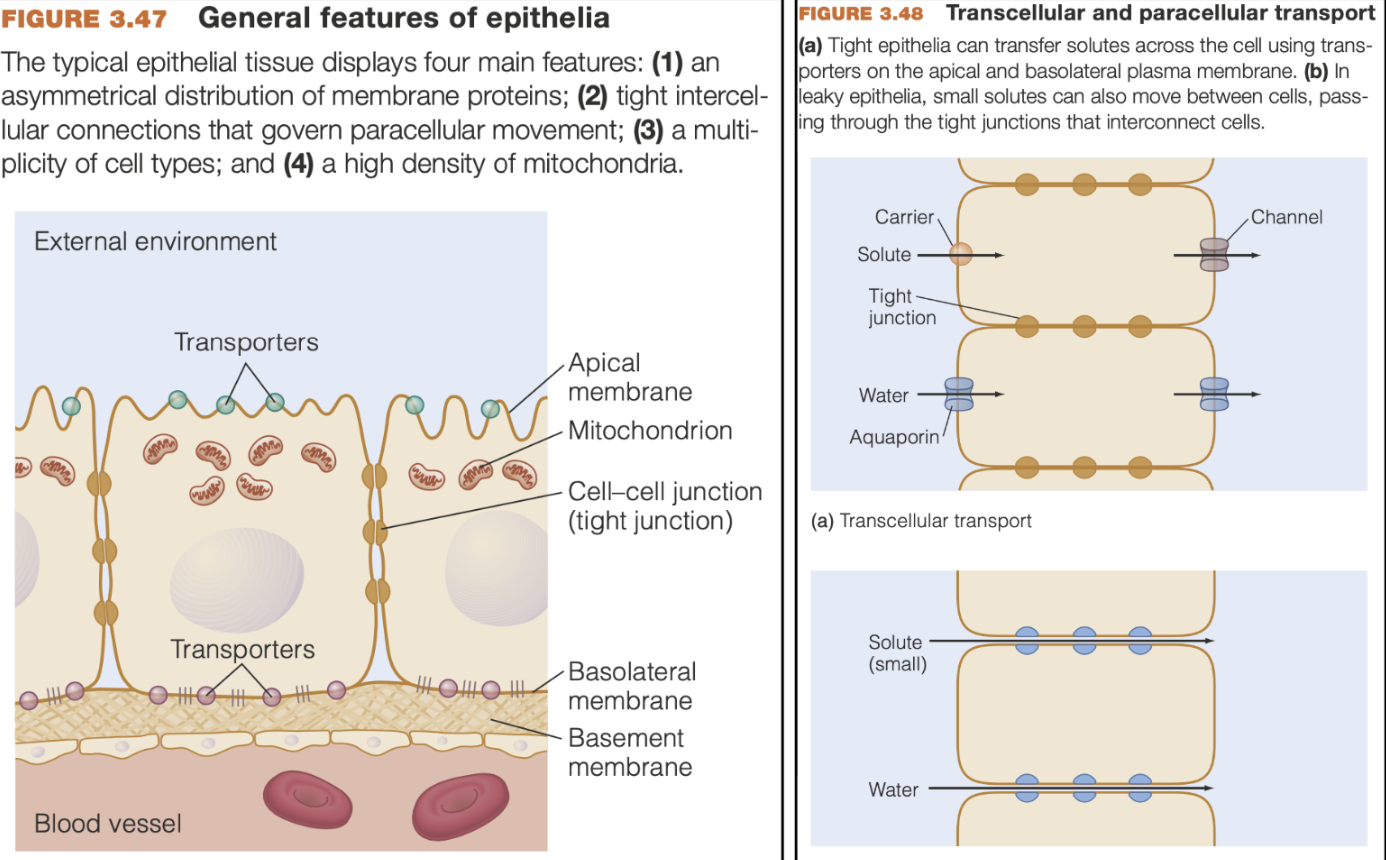
Epithelial cells* (be familiar)
Epithelial cell function depends on the asymmetrical distribution of transporters within the cell
Apical cell membrane - exposed to the outside
Basolateral cell membrane - faces inward
They are interconnected by protein linkages - tight junctions
Limits movement of solutes and water around cells, and restricting free movement of the membrane proteins between the apical and basolateral regions
Ion transport demands lots of energy - Epithelial cells have abundant mitochondria to product ATP
Transcellular transport - the movement of solutes (or water) through epithelial cells
Paracellular transport - the movement of solutes (or water) between adjacent cells)
Epithelial cells can control movement in/out of an organ
Small molecules (water, ions) can cross through the membrane connections/tight junctions via paracellular transport, though large molecules cannot
Leaky epithelia - permit paracellular transport
Tight epithelia - minimal paracellular transport
Tight Junctions
Impermeable barrier
In between 2 sets of cells (like epithelial cells)
Prevents paracellular movement (between 2 cells; few molecules selective), so molecules can only do transcellular movement (through a cell) if permitted
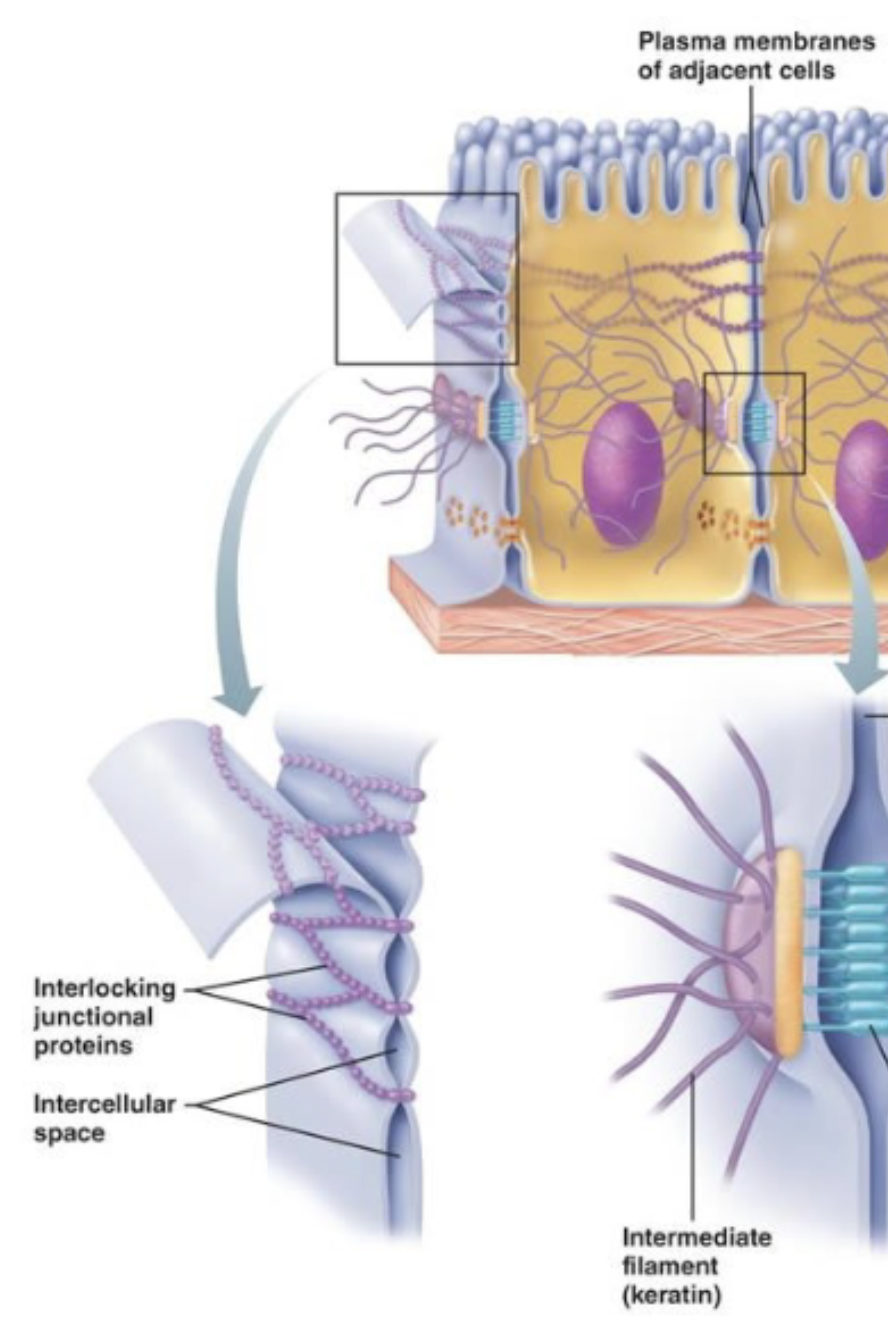
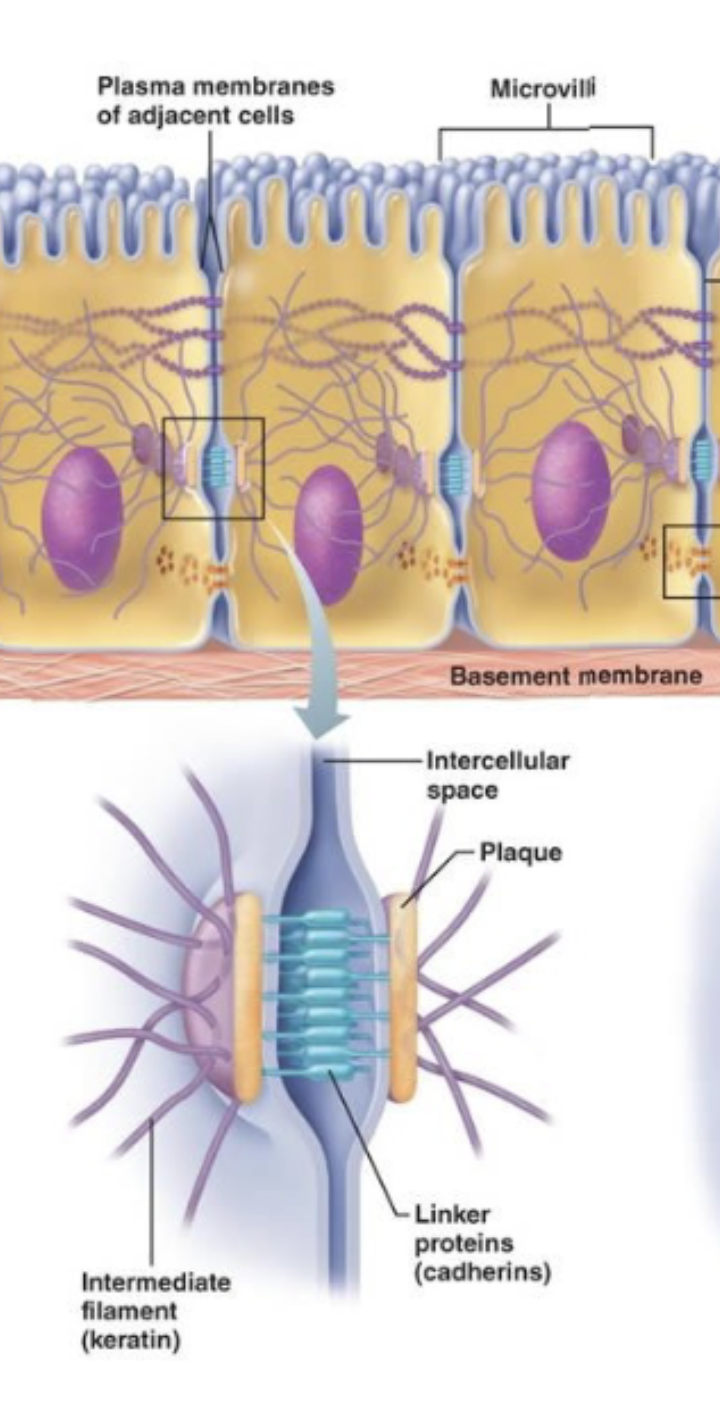
Desmosomes (spots) / Adheren junctions (band, velco-like)
For anchoring
Desmosomes - keep cells from separating apart, very strong and rigid
Tend to be in tension-bearing organs
Skin, heart, bladder, esophagus
Adherens - Hold cells together while allowing some flexibility
Tend to be in flexible tissues/organs
Intestinal lining, blood vessels
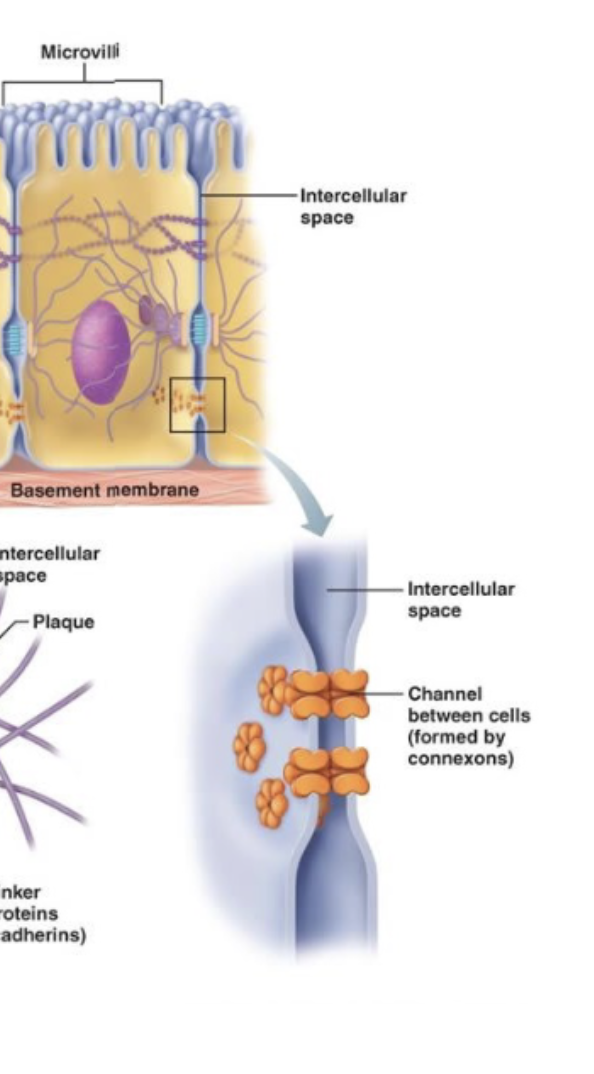
Gap junctions
Communication
Tunnels that allow movement of chemicals or charge from one cell to another
Cell to cell
Like in cardiac cells (charge)
Plasma membrane structure
Phospholipids - polarity
Polar, hydrophilic phosphate group heat
Nonpolar, hydrophobic fatty acid tails
Channel proteins
Peripheral proteins - receptors, tags
Glycoproteins
Cholesterol - temperature / thermal regulation
Plasma membrane relative permeability / diffusion through plasma membrane
1) Hydrophobic molecules (nonpolar)
Easily pass through via diffusion
O2, CO2, N2 → small, nonpolar
Steroids
Testosterone, cortisol, estrogen, Vitamins A/E/D
Large nonpolar/hydrophobic molecules can pass through
2) Small, uncharged polar molecule
Glycerol
3) Large, uncharged polar molecules
Unless hydrophobic (nonpolar), big molecules have a harder time passing through
Glucose, sucrose, slightly impermeable
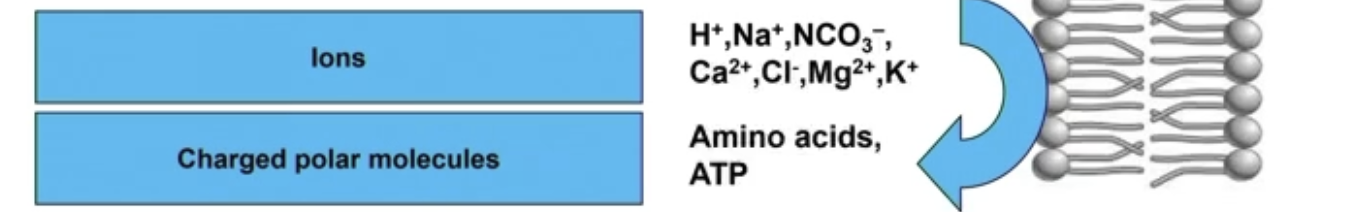
4) Ions
Charge = very difficult to pass through
H+, Na+, K+, etc
4) Charged polar molecules
Amino acids, ATP
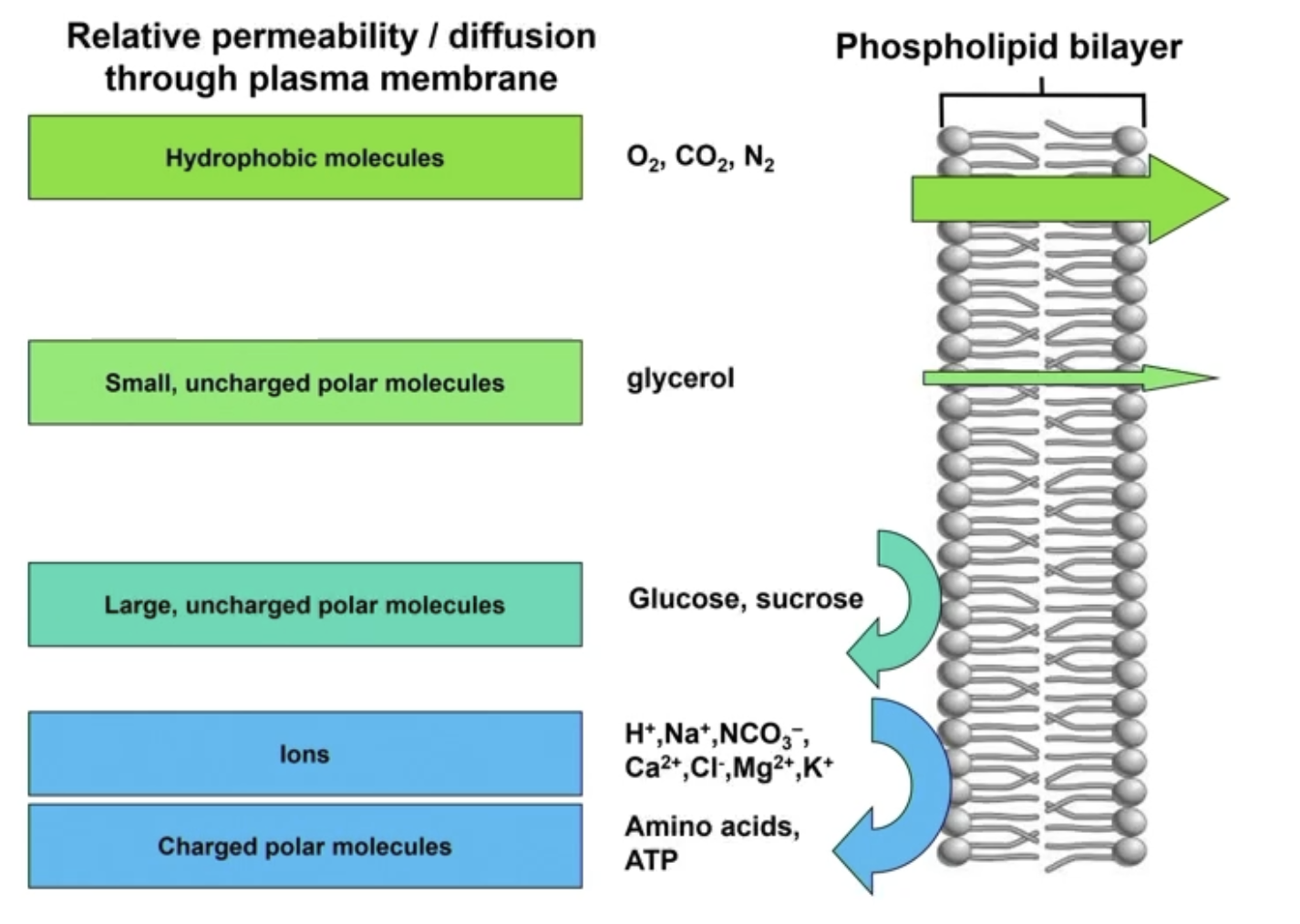
Molecules that can EASILY DIFFUSE through the phospholipid bilayer
1) Hydrophobic (nonpolar) molecules
O2, CO2, N2
Steroids
2) Small, uncharged polar molecules
Glycerol
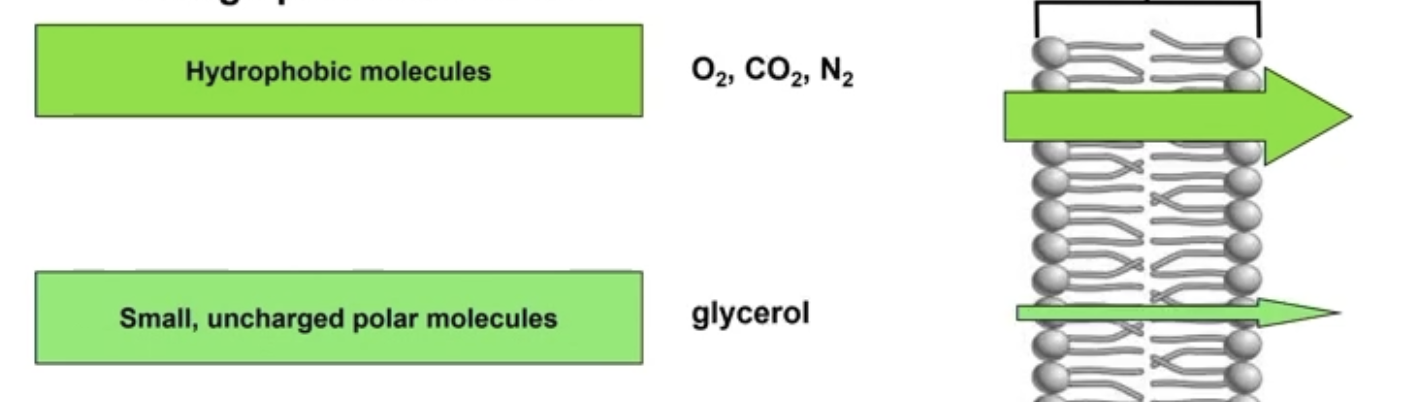
Molecules that HAVE DIFFICULTY DIFFUSING through the PM
1) Large, uncharged polar molecules
Glucose, sucrose
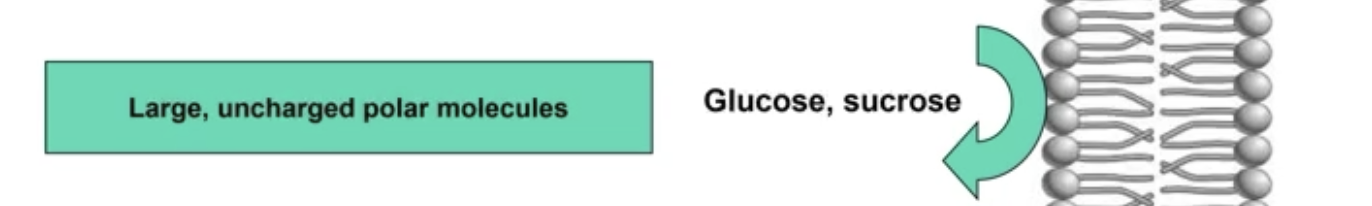
Molecules that are IMPERMEABLE to the PM
1) Ions
Na+, Ca2+, Cl-
2) Charged polar molecules
Amino acids, ATP
Movement of molecules across the PM
Simple (passive diffusion)
Through PM
Facilitated diffusion
Through Protein channels
Active Transport
Through protein channels, ATP
Bulk Transport
Simple Diffusion
Concentrated area to less concentrated region to reach equilibrium through time
Electrochemical gradients
Form of energy storage
Random walk process due to random thermal motion
Molecules collide with other molecules and change direction unpredictably (random direction and length), but the net diffusion is from high to low concentration
This produces a random walk.
Small and nonpolar molecules can diffuse through the PM
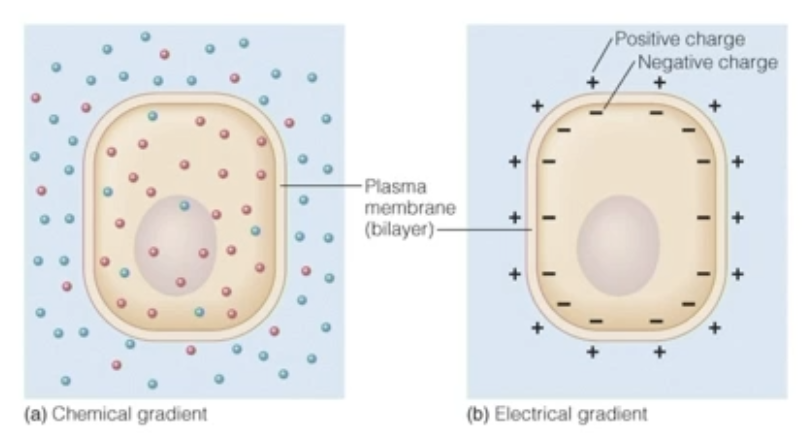
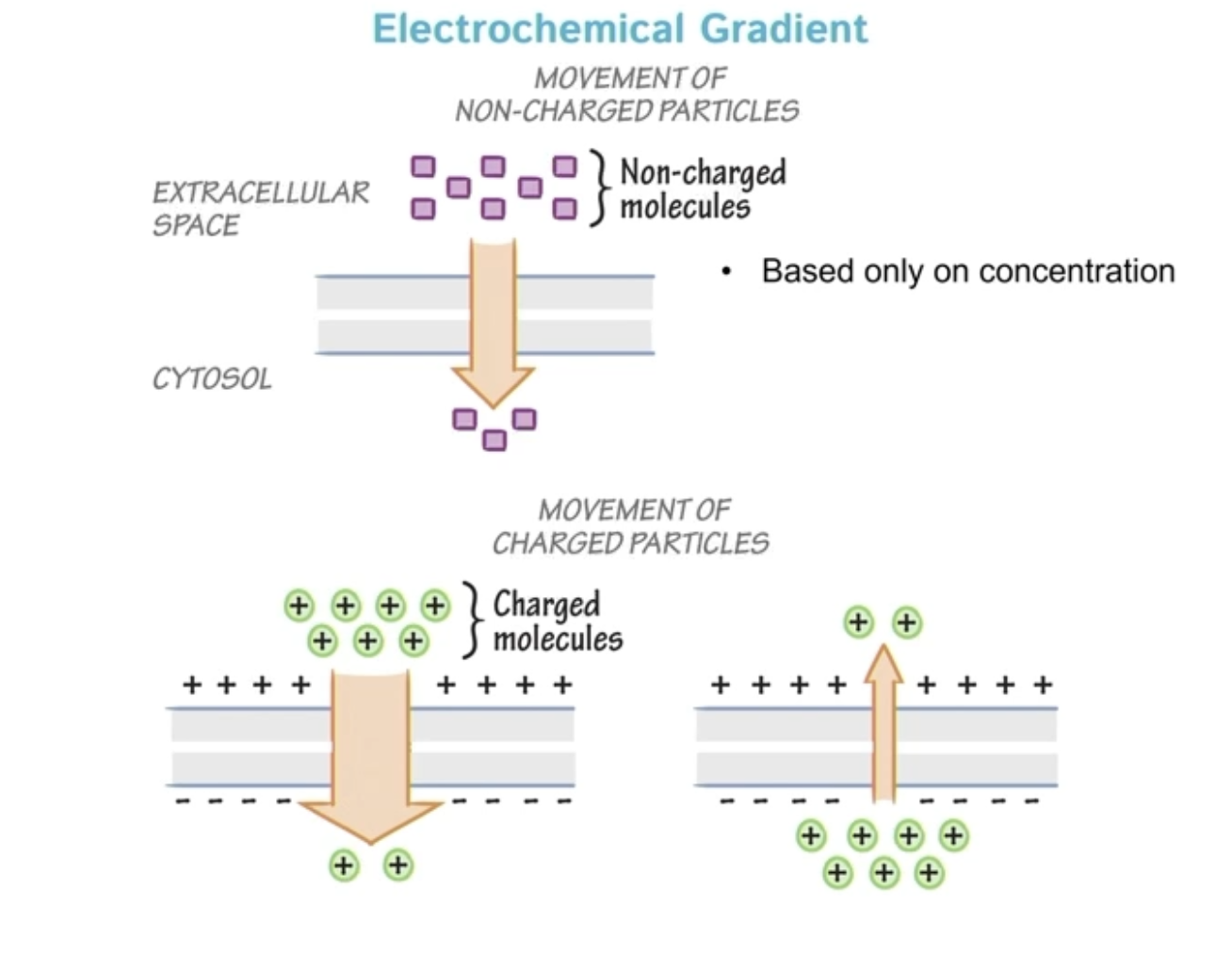
Electrochemical gradient
Gradients can be chemical, electrical, or both
Non-charged molecules only have movement due to concentration, so they diffuse independent of membrane charge
Form of energy storage (potential energy)
In electrochemical gradients:
Ions are held apart by membranes
Their movement is restricted
Energy is stored in:
Unequal concentrations
Separated charges
When channels open, ions move spontaneously, releasing that stored energy.
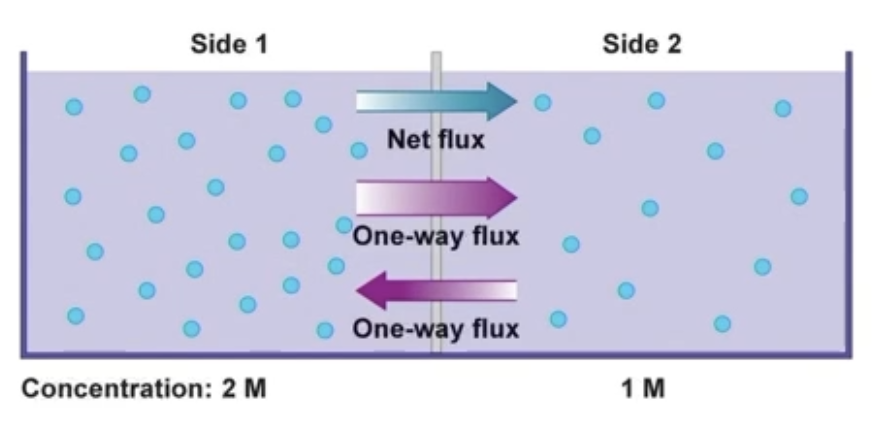
Flux & Net flux
Flux - measure of diffusion rate
Net Flux - difference between the 2 one-way fluxes
Measure of net gain of molecules by one side and net loss form the other side
Diffusion still occurs while net flux = 0 ; equal movement in and out
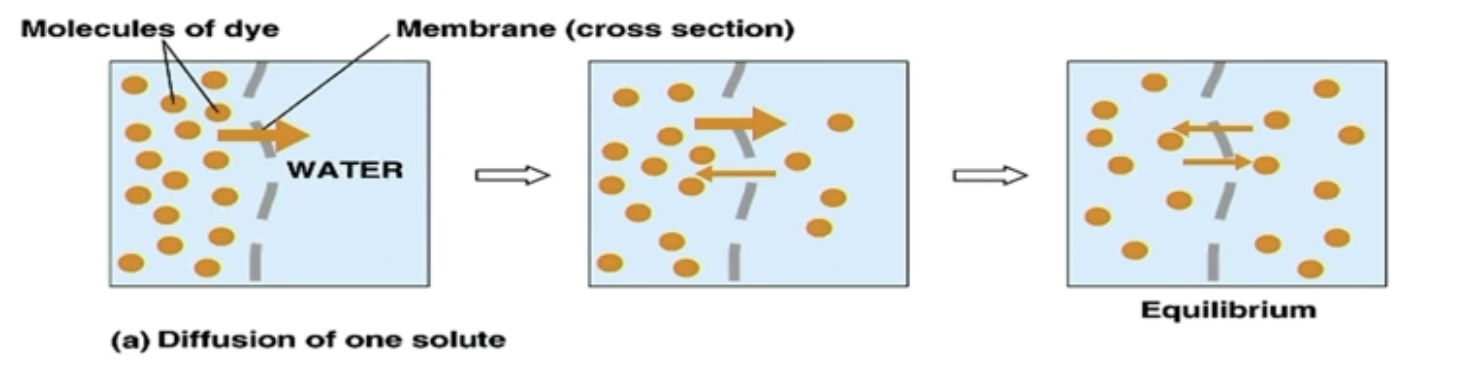
Passive Diffusion (for 1 substance) *NO CHARGE
Movement of molecules due to the intrinsic kinetic energy of molecules
No metabolic energy (ATP) used - Passive
Movement from higher to lower concentration
At equilibrium, Net flux (Rate of diffusion) = 0
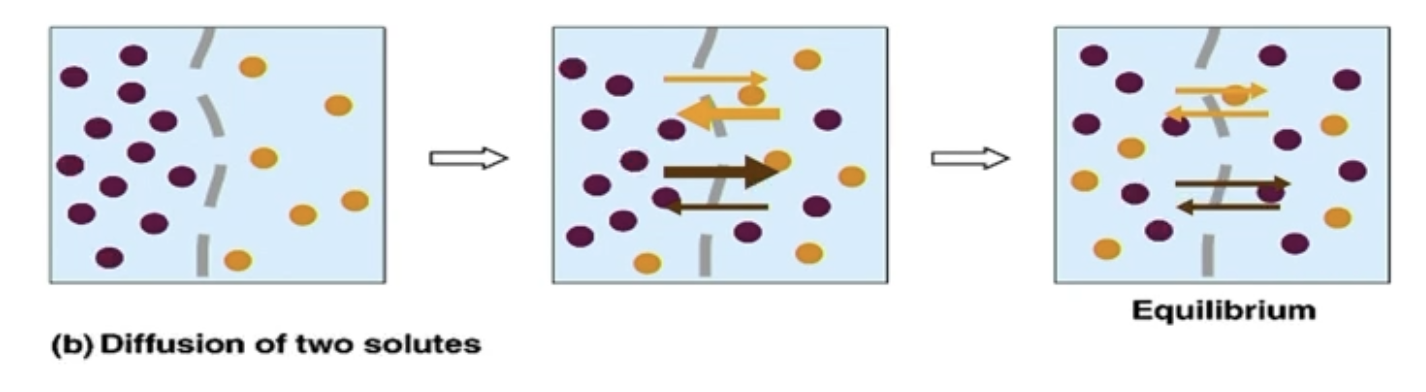
Passive Diffusion (for 2 substances)
Each substance diffuses down its OWN concentration gradient, independent of the concentration gradients of other substances
At equilibrium, equal number of each molecule on either side
Passive Diffusion - Net Flux direction and magnitude
Net flux direction and magnitude depend on:
Permeability
Change by opening and closing channels
Concentration gradient
Temperature
Higher T = higher diffusion rate
Surface area
Big SA increase diffusion
Size of molecule
Smaller molecules faster than bigger
Distance
How thick the membrane is, how far a molecule has to diffuse
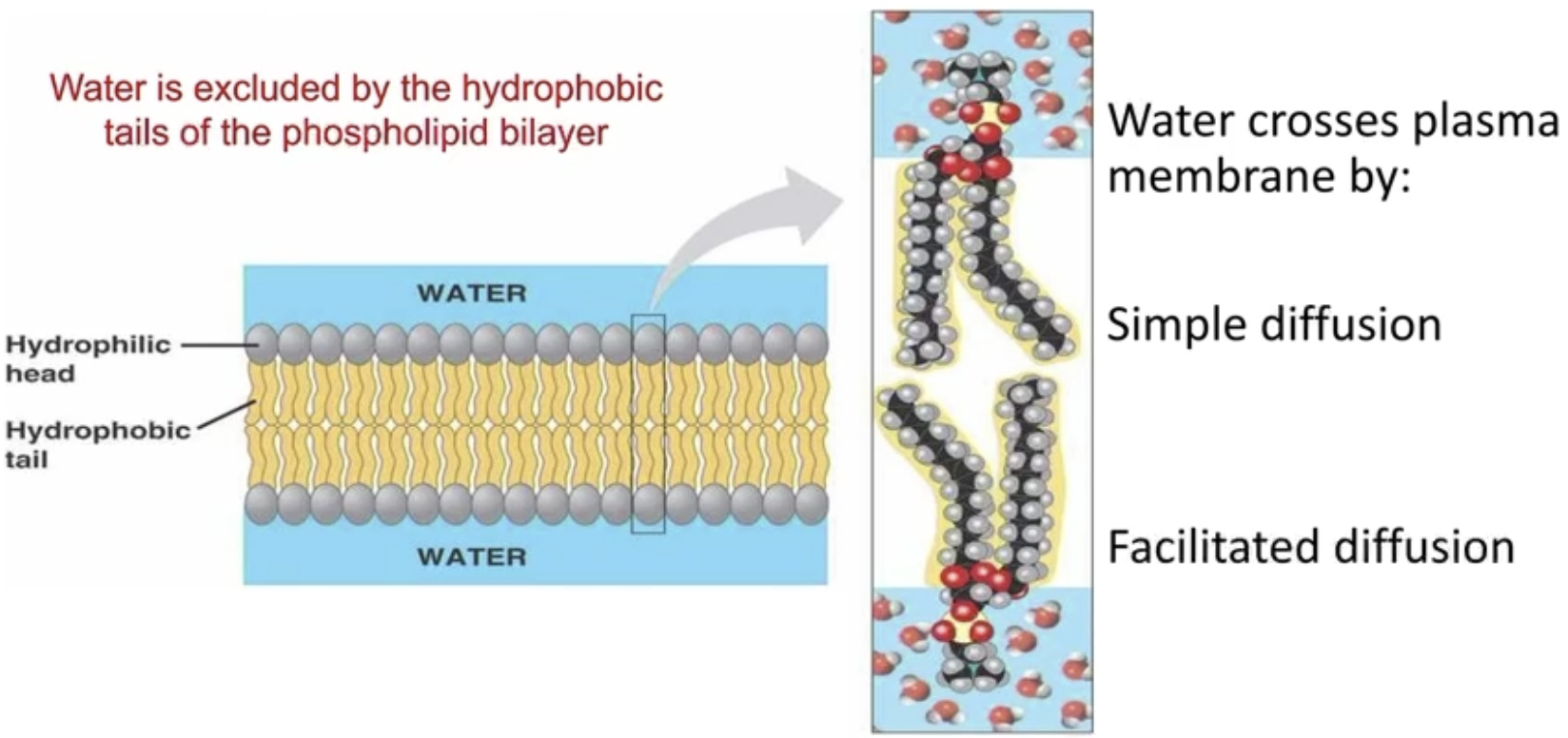
Water and PM Diffusion
Water is excluded by the hydrophobic tails of the phospholipid bilayer
Can diffuse, but not via simple → FACILITATED
Majority of water diffusion is via aquaporins (facilitated), only a bit through PM (Simple)
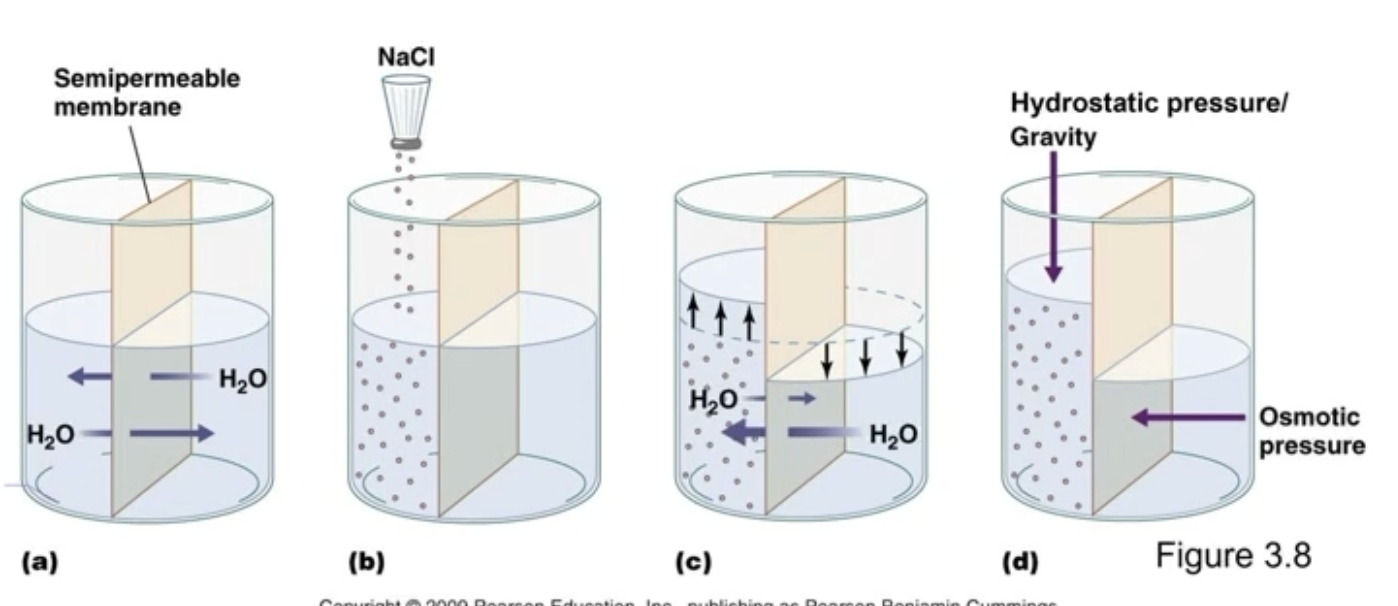
Osmosis
Passive diffusion of water
Net diffusion of water from a region of high water concentration to a region of low water concentration
Facilitated by aquaporins
Osmolarity
Total concentration of solutes in a solution
Depends on the total number of molecules, not individual type
Isosmotic
Hyperosmotic
Hyposmotic
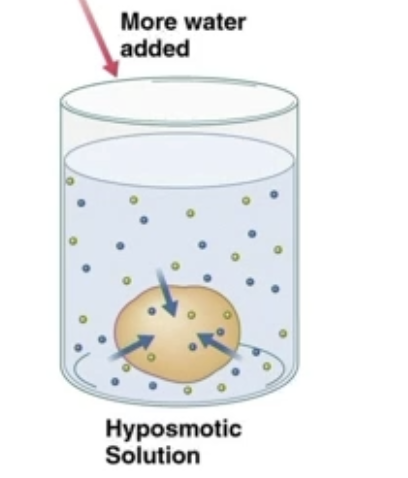
Hyposmotic solution
Solution has less solute than another solvent
If solution is hyposmotic to the sample, the sample is hyperosmotic to the solution, causing the sample to swell
Sample will shrink if it is hyposomotic to the solution
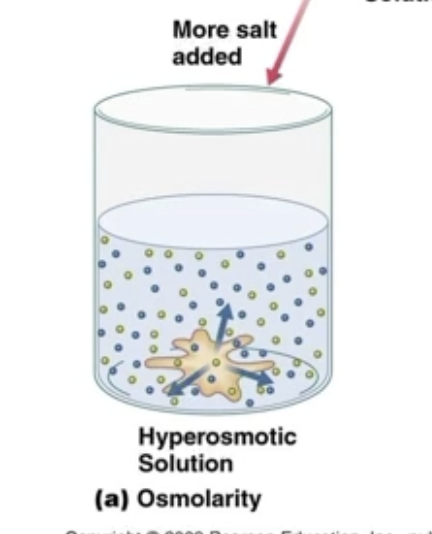
Hyperosmotic
Solution has higher concentration that another solvent
If solution is hyperosmotic to the sample, the sample is hypoosmotic to the solution, causing the sample to shrink
Sample will swell if it is hyperosmotic to the solution
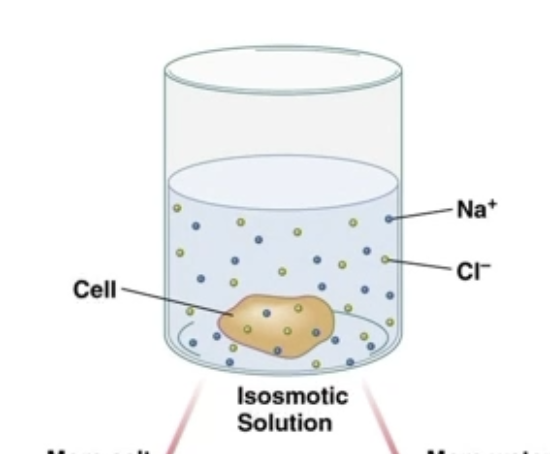
Isosmotic
2 solutions have same concentration of solutes
Solutes don’t need to be the same
Sample will stay the same size
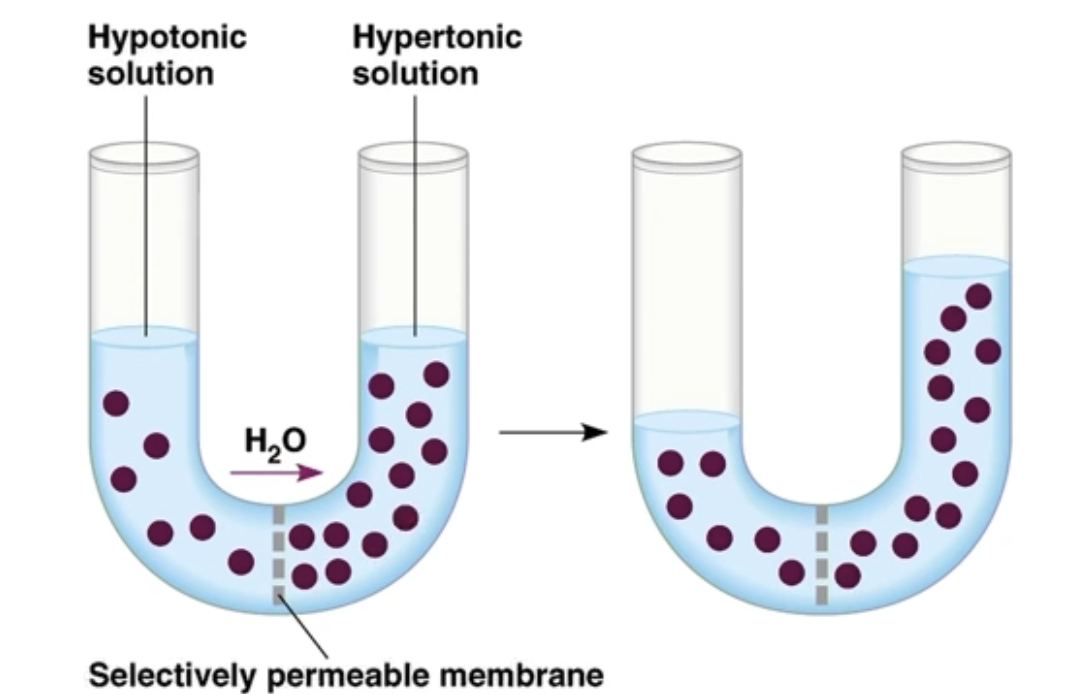
Direction of water movement through a permeable membrane
Water will move to the hypertonic side, against its concentration gradient, to decrease the salt gradient
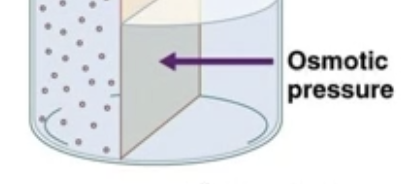
Osmotic Pressure
Pressure generated by water moving based on osmolarity
Osmotic pressure is the pulling force that draws water into a compartment because of solute concentration differences across a semipermeable membrane.
(Why water wants to move)
Hydrostatic Pressure
Pressure exerted by the standing column of water - gravity
The pushing force exerted by a fluid on the walls of its container or membrane; caused by gravity
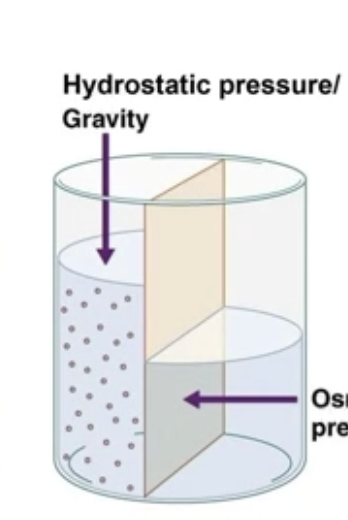
Osmotic pressure and Hydrostatic pressure
Water would want to move through an impermeable membrane forever towards the more concentrated side due to osmotic pressure, but it cannot, due to external hydrostatic pressure (gravity)