7.1-7.3: Energetic of a Reaction and Energy in Reactions
During a chemical reaction there is always an energy change.
Exothermic Reactions
^^Exothermic reactions give out energy, therefore there is a temperature rise^^
These reactions can be described as:
Reactants ———> Products + energy
In exothermic reactions energy is transferred to the surroundings so the temperature of the surroundings increases
This energy is transferred from the chemical energy store of the chemical system to the surroundings and so the energy of the system falls - this means that the energy change is negative
The overall transfer is from the %%system to the surroundings%%
@@Combustion, oxidation, and neutralisation reactions are typical exothermic reactions@@
Hand warmers used in the wintertime are based on the release of heat from an exothermic reaction
Self-heating cans of food and drinks such as coffee and hot chocolate also use exothermic reactions in the bases of the containers
mixing silver nitrate and sodium chloride solutions gives a white precipitate of silver chloride- and a temperature rise
EXothermic reactions heat Exits the system
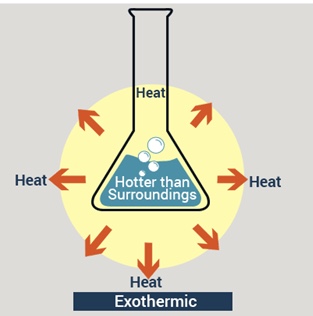
Overall Exothermic Reaction
\
If more energy is released than is absorbed, then the reaction is exothermic
More energy is released when new bonds are formed than energy required to break the bonds in the reactants
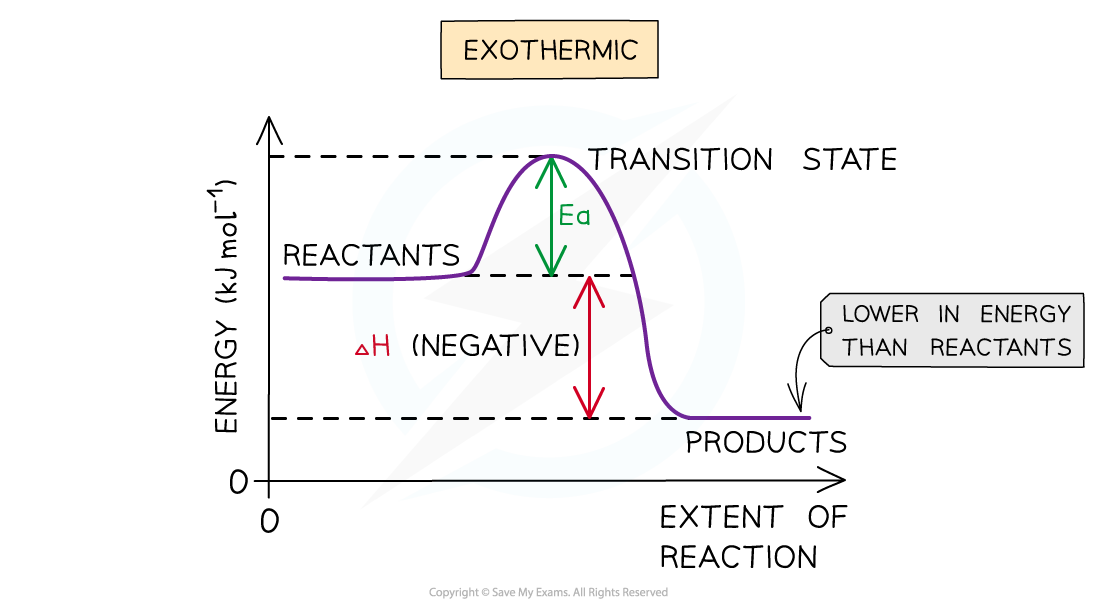
The change in energy is negative since the products have less energy than the reactants
Therefore an exothermic reaction has a negative ΔH value
\
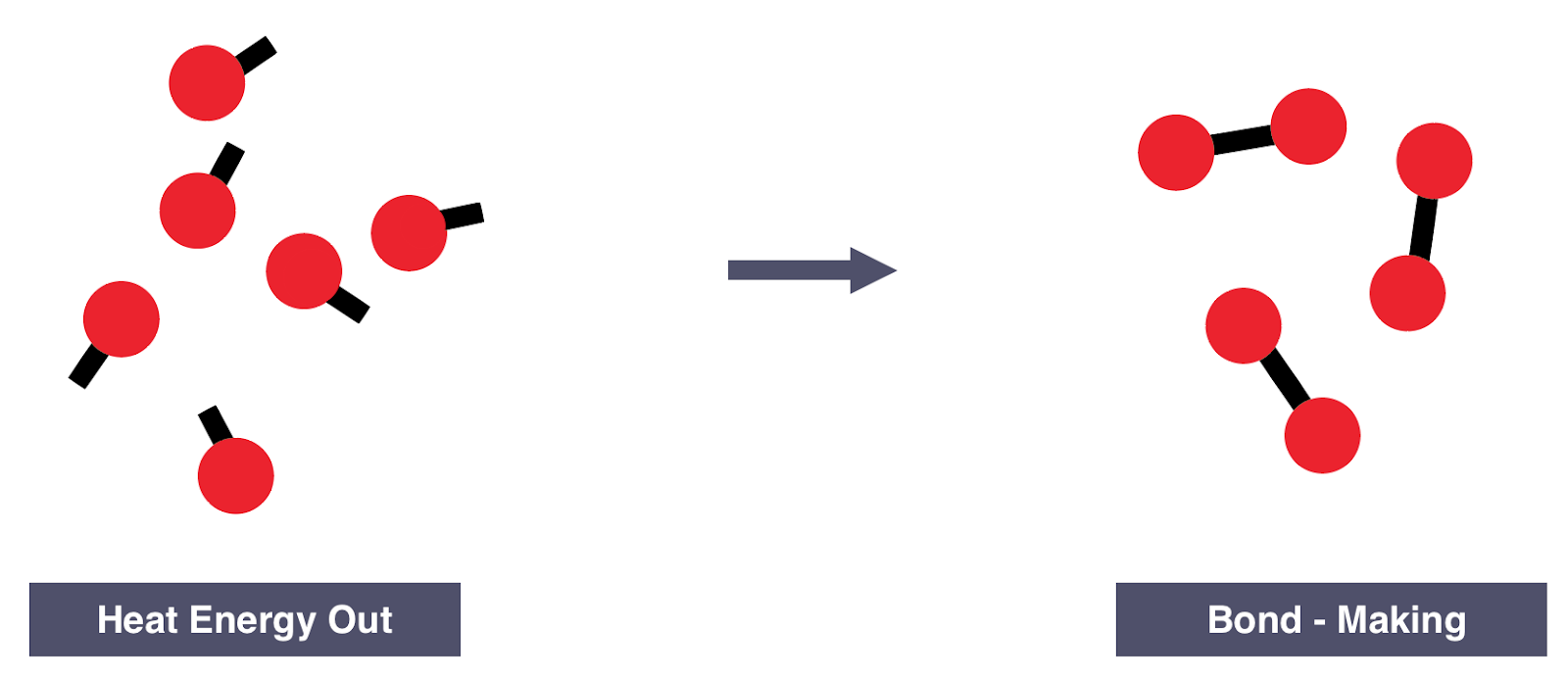
- During an exothermic reaction, more energy is energy is given out when new bonds are made than the energy taken in to break bonds
- This means that the energy of the products will be lower than the energy of the reactants, so the change in enthalpy (ΔH) is negative
- This is represented on the energy-level diagram above with the energy of the products being lower than that of the reactants
Endothermic Reactions
- %%Endothermic reactions take in energy from their surroundings.%%
- These reactions can be described as:
- Reactants + Energy ———> Products
- In endothermic reactions energy is taken in from the surroundings so the temperature of the surroundings decreases
- This energy is transferred to the chemical energy store of the chemical system and so the energy of the system increases - this means the energy change is positive
- The overall transfer is from the surroundings to the system
These types of reactions are much less common than the exothermic reactions
Electrolysis, thermal decomposition reactions and the first stages of photosynthesis are typical endothermic reactions
Sports injury treatments often use cold packs based on endothermic reactions to take heat away from a recently injured area to prevent swelling
Sherbet us Citric acid plus the base sodium hydrogen carbonate. The neutralization that occurs takes in heat- so your tongue cools.
ENdothermic reactions heat ENters the system
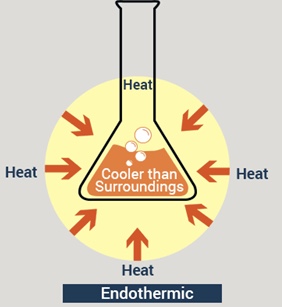
Overall Endothermic Reactions
- If more energy is absorbed to break bonds than is released to form new bonds, this reaction is endothermic overall
- The change in energy is positive since the products have more energy than the reactants
- The symbol ΔH (delta H) is used to show the change in heat energy. H is the symbol for enthaply, which is a measure of the total heat of reaction of a chemical reaction
- Therefore an endothermic reaction has a positive ΔH value, which is shown on the energy level diagrams and in calculations
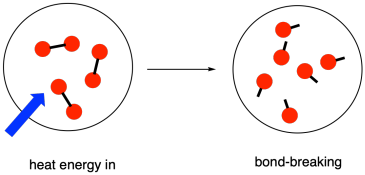
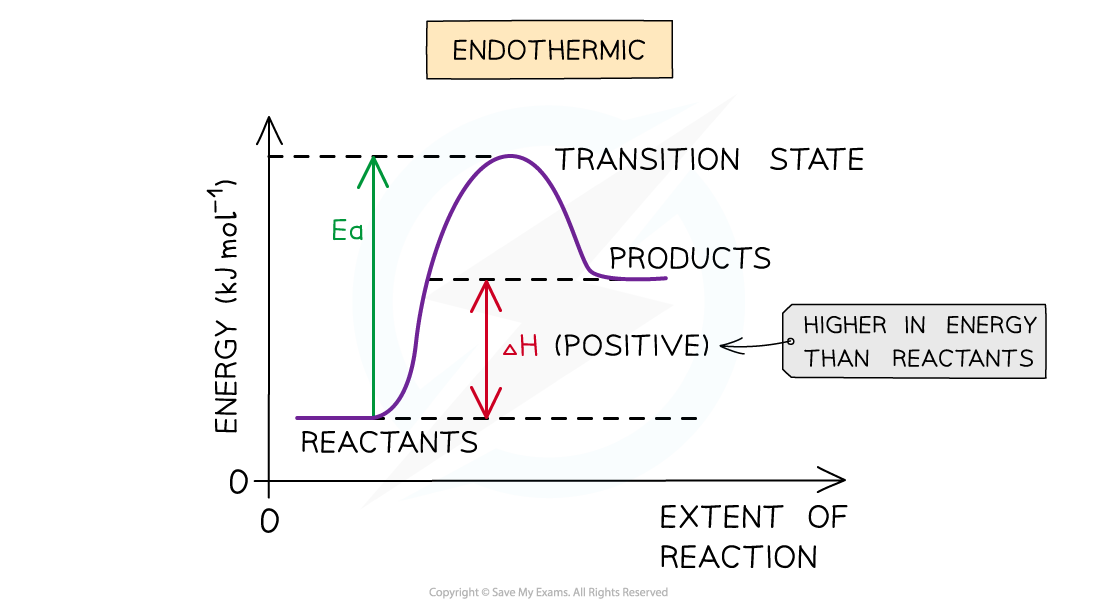
Energy level diagram a little overview
- Energy level diagrams (sometimes called reaction pathway diagrams or reaction profiles) are graphical representations of the relative energies of the reactants and products in chemical reactions
- The energy of the reactants and products are displayed on the y-axis and the reaction pathway (a bit like time) is shown on the x-axis
- The difference in height between the energy of reactants and products represents the overall energy change of a reaction.
- This is usually a sketch but can be drawn to scale if data is provided
Calculating Energy of Reaction
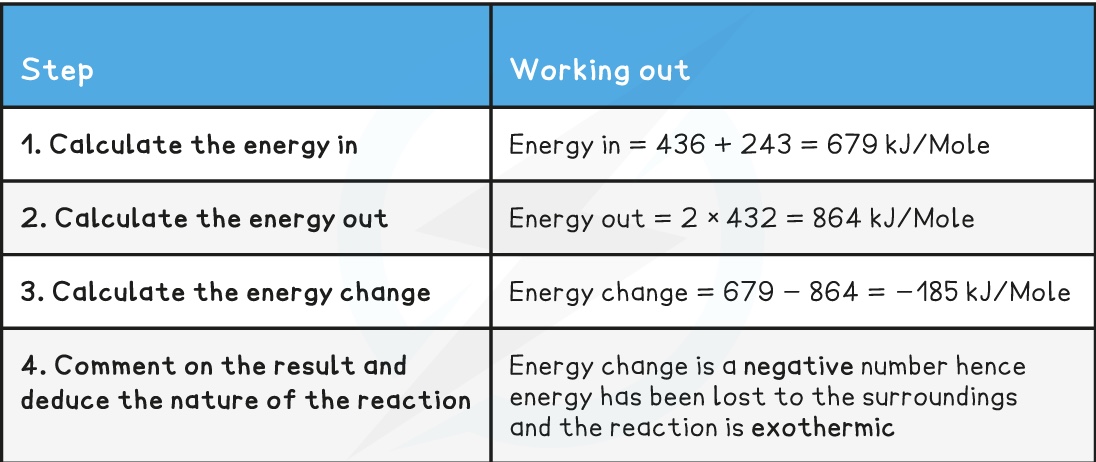
^^The bond energy is the energy needed to break bonds, or released when these bonds form. It is given in kJ/mole.^^
- Each chemical bond has a specific bond energy associated with it
- This is the amount of energy required to break the bond or the amount of energy given out when the bond is formed
- This energy can be used to calculate how much heat would be released or absorbed in a reaction
- To do this it is necessary to know the bonds present in both the reactants and products
Method
- Write a balanced equation if none is present already
- Optional - draw the displayed formula in order to identify the type and number of bonds more easily
- Add together all the bond energies for all the bonds in the reactants – this is the ‘energy in’
- Add together the bond energies for all the bonds in the products – this is the ‘energy out’
Calculate the energy change:
equation : Energy change = energy in - energy out

Energy Transfer
What is a fuel?
- ==A fuel is any substance we use to provide energy.==
- We convert the chemical energy in the fuel into another form of energy we burn most fuels, to obtain their energy in the form of heat.
- The fossil fuels
- The fossil fuels- coal petroleum (oil) and natural gas (methane) are the main fuels used around the world. We burn to release heat.
- A fuel is a substance which releases energy when burned
- When the fuel is a hydrocarbon then water and carbon dioxide are produced in complete combustion reactions
- Propane for example undergoes complete combustion according to the following equation:
C3H8 + 5O2 → 3CO2 + 4H2O ΔH = -2219 kJ/mol
So what makes a good fuel?
- How much heat does it give out? we want as much heat as possible per tone of fuel.
- Does it cause pollution? if it causes A LOT of pollution we may be better off without it!
- Is it easily available? we need a steady and reliable supply.
- Is it easy and safe to transport and store? most fuels catch fire quite easily so safety is always an issue
- How much does it cost? The cheaper the better.
- The fossil fuels give out a lot of heat but they cause pollution, with coal the worst culprit, the pollutants in key carbon dioxide which is linked to global warming and other gases that cause acid rain we will look into this later on..
Two fuels growing in importance
Ethanol
Hydrogen
Hydrogen as a Fuel
- Hydrogen is used in rocket engines and in fuel cells to power some cars
- Hydrogen has a some unique advantages and disadvantages regarding its use as a fuel:
- Advantages:
- It releases more energy per kilogram than any other fuel (except for nuclear fuels)
- It does not pollute as it only produces water on combustion, no other product is formed
- Disadvantages:
- Expensive to produce and requires energy for the production process
- Difficult and dangerous to store and move around (usually stored as liquid hydrogen in highly pressurised containers) as it is so flammable it easily explodes when stored under pressure
Nuclear Fuels ☢️
Nuclear Fuels are not burned. They contain unstable atoms called Radioisotopes over time these break down naturally into new atoms give out radiation and a-lot of energy.
But you can also force radioisotopes to breakdown by shooting neutrons at them. That is what happens in a nuclear power station.
The energy given out is used to heat water to make steam. Jets of steam are then used to drive turbines for generating electricity.
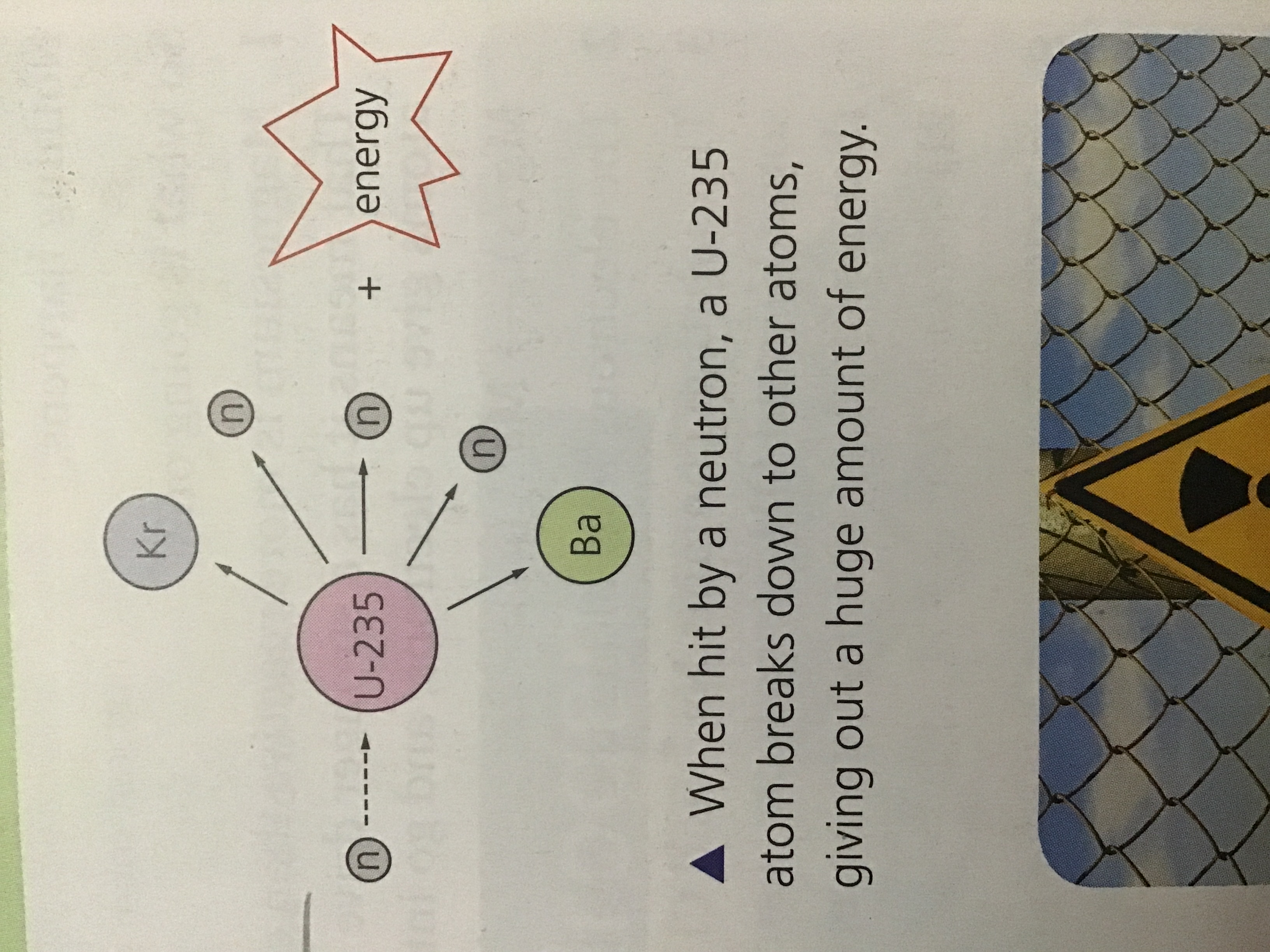
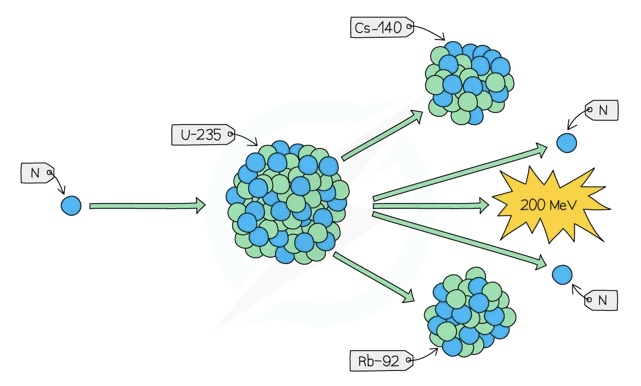
Uranium-235 undergoes decay and gives off heat energy which nuclear power stations harness, ( Uranium-235 is often used. When it decays, the new atoms that form are also unstable and break down further)
The heat it produces is used to heat water to steam, which in turn is used to power turbines to generate electricity as we mentioned
Nuclear fuel energy is clean as it does not produce pollutants such as CO2 or oxides of nitrogen or sulfur
It gives out huge amounts of energy, a pellet of nucleus fuel the size of a pea can give as much energy as a tone of coal.
No CO2 or other polluting gases are formed.
disadvantage = But nuclear power plants are expensive to build and maintain as well as being potentially dangerous in the event of an accident as radioactive materials may be released
disadvantages = Radioactive waste still needs to be safely stored so that it does not contaminate the local ecosystems
Hydrogen Fuel cells
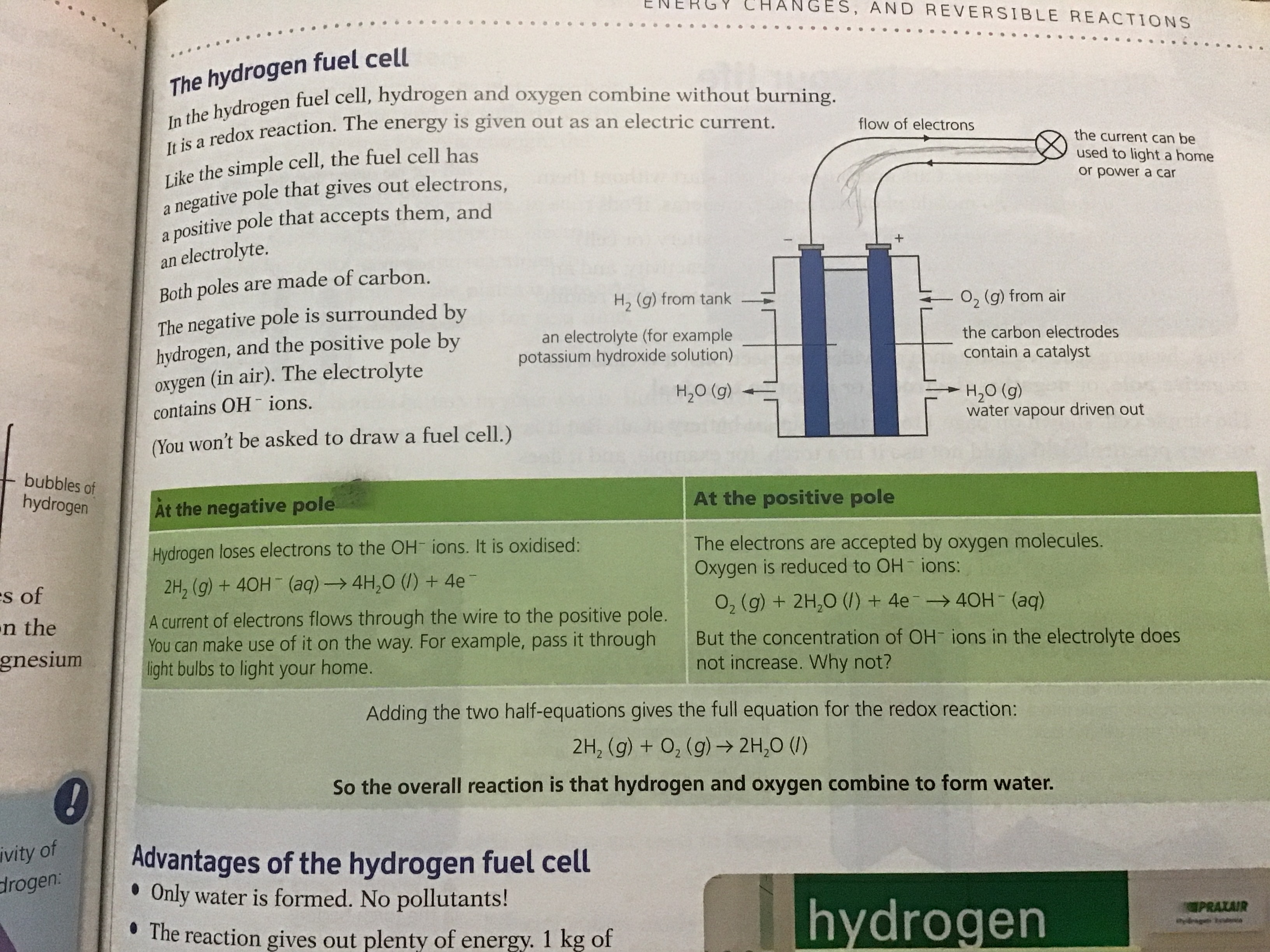
- A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode
- This keeps the hydrogen and oxygen separate, reducing the hazards associated with combusion of hydrogen
- The only product is water
- These cells are becoming more common in the automotive industry to replace petrol or diesel engines
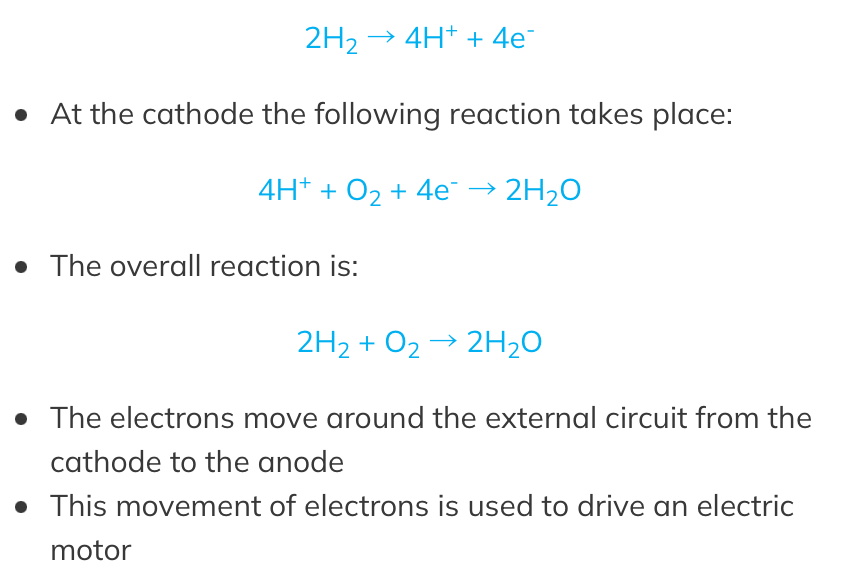
- H2 and O2 are pumped through two porous electrodeswhere the half-reactions occur
- The following reaction occurs at the anode:
- This keeps the hydrogen and oxygen separate, reducing the hazards associated with combusion of hydrogen
- The only product is water
- These cells are becoming more common in the automotive industry to replace petrol or diesel engines
- H2 and O2 are pumped through two porous electrodeswhere the half-reactions occur
- The following reaction occurs at the anode:
Advantages
They do not produce any pollution
They produce more energy per kilogram than either petrol or diesel
No power is lost on transmission as there are far fewer moving parts than in an internal combustion engine
Fuel cells are quiet and lightweight compared to combustion engines
Disadvantages
- Materials used in producing fuel cells are expensive
- High-pressure tanks are needed to store the oxygen and hydrogen in sufficient amounts
- Fuel cells are affected by low temperatures, becoming less efficient
- Hydrogen is expensive to produce and store
\
\