chem unit 6 - chemical bonds
1/21
Earn XP
Description and Tags
* don't need to know angles
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
chemical bonds and octet rule
attractive force between atoms or ions that binds them together as one unit
bonds form to increase stability (octet rule - atoms want 8 valence electrons)
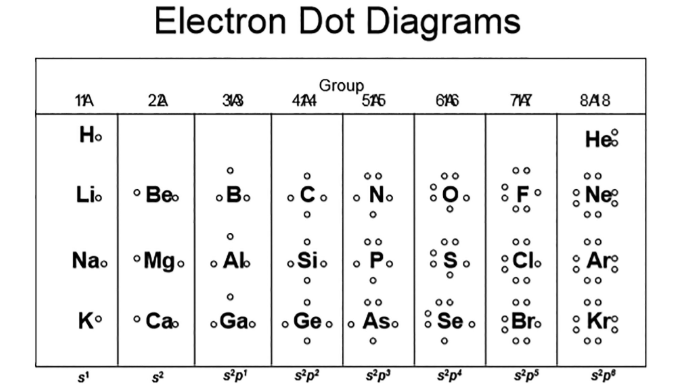
only valence electrons
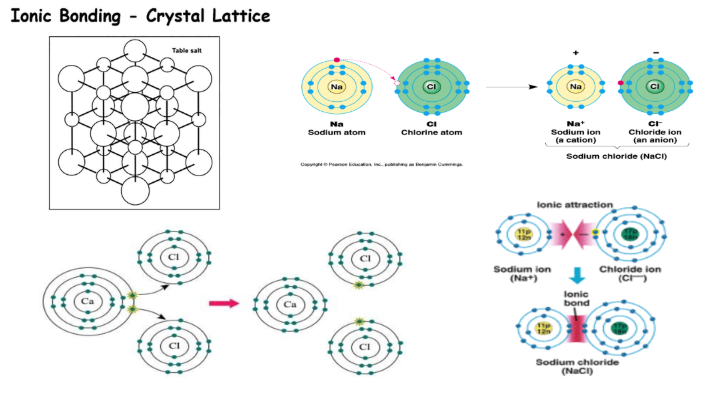
ionic bond properties
metal + nonmetal
electrons transferred from metal to nonmetal
structure: crystal lattice
state: solid (hard/brittle)
melting point: high
soluble in water: yes
electric conductivity: yes if solution or molten
covalent bond properties
nonmetal + nonmetal
a pair of electrons is shared between atoms
structure: true molecules
state: liquid or gas
melting point: low
soluble in water: not usually
electric conductivity: no
other properties: odorous
electron dot diagrams
crystal lattice
covalent bonding
can form single (2e-), double (4e-), or triple (6e-) bonds
unshared pairs are pairs of unbonded valence electrons
each atom still needs a full outer shell
drawing covalent lewis dot structures
count total valence electrons
connect all atoms with single bonds
“single” atom is center
“multiple” atoms on the outside
C is always the center, H is always outside
check for multiple centers
full octet? if not then add multiple bonds
extra electrons? put on central atom
usual number of bonds for C, O, H, N, and halogens
C - 4
O - 2
H - 1
N - 3
halogens - 1
octet exceptions
H - 2
Be - 4
B - 6
P - 10
S - 12
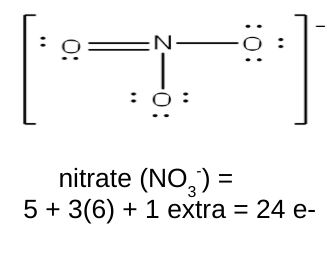
polyatomic ions
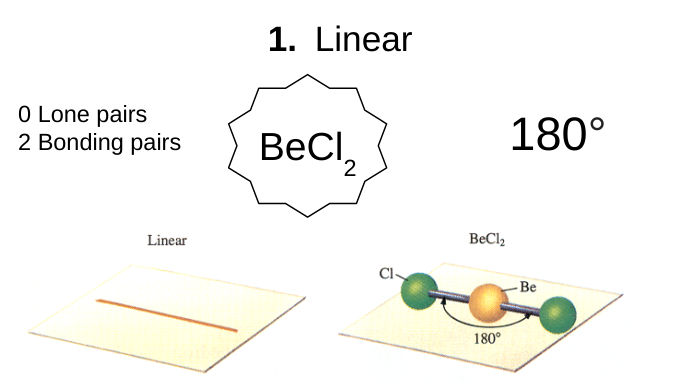
linear
0 lone pairs
2 bonding pairs
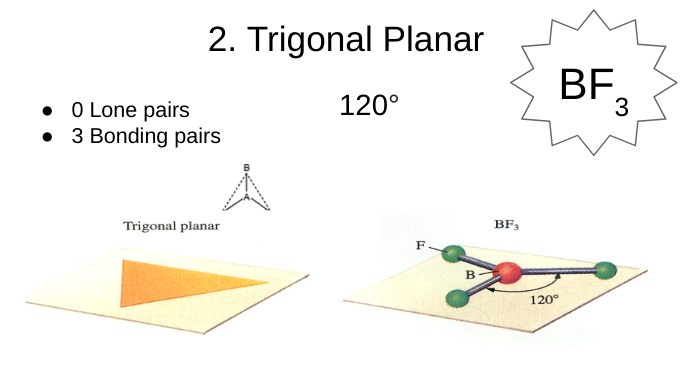
trigonal planar
0 lone pairs
3 bonding pairs
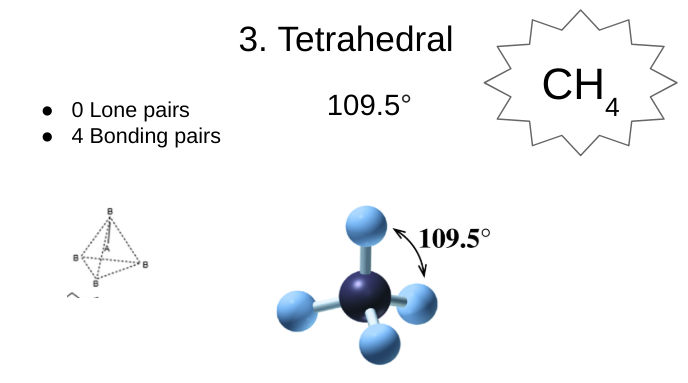
tetrahedral
0 lone pairs
4 bonding pairs
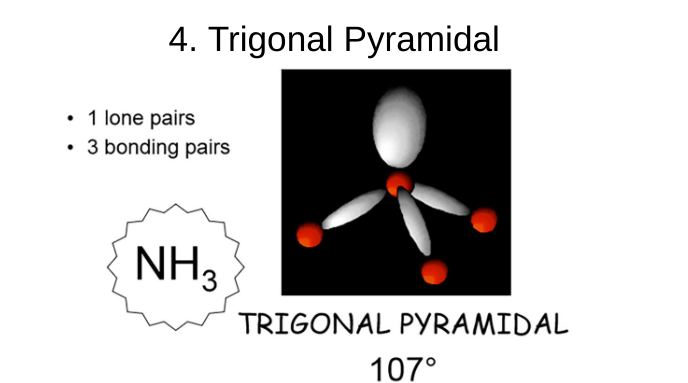
trigonal pyramidal
1 lone pair
3 bonding pairs
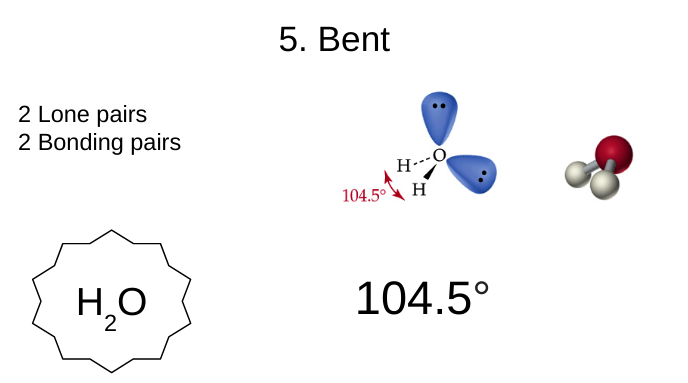
bent
2 lone pairs
2 bonding pairs
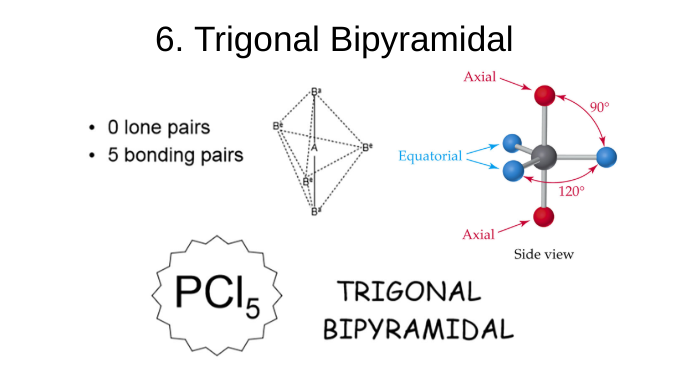
trigonal bipyramidal
0 lone pairs
5 bonding pairs
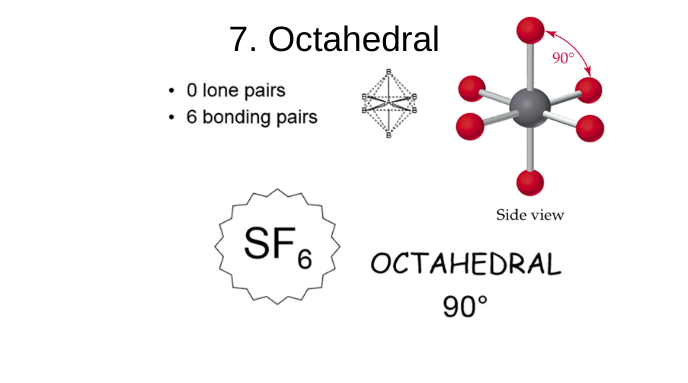
octahedral
0 lone pairs
6 bonding pairs
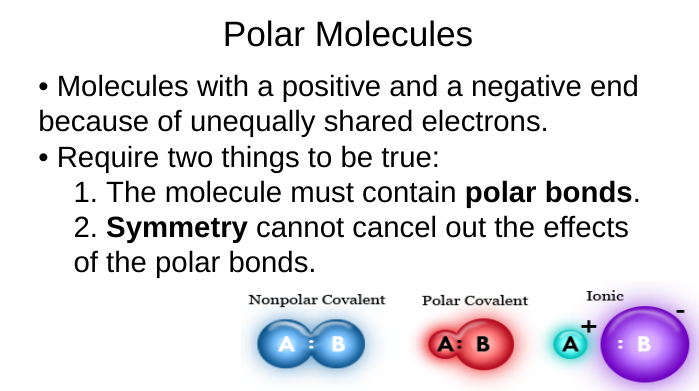
polar molecules
unequally share electrons
2 requirements:
polar bonds
symmetry can’t cancel out polar bond effects
bond polarity
0-0.4 nonpolar
0.5-2.0 polar covalent
2.1-3.3 ionic
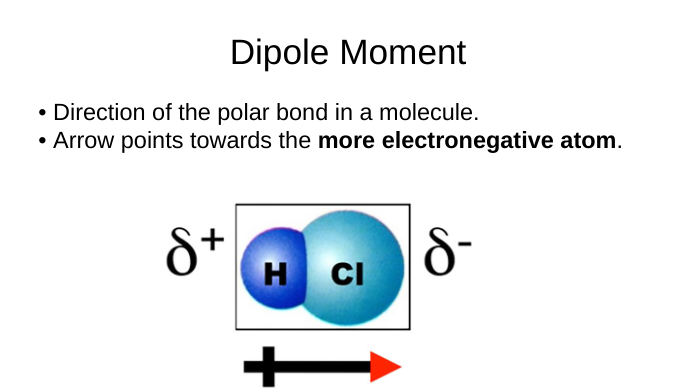
dipole
arrow points towards the more electronegative atom
symmetry
nonpolar molecules are symmetrical
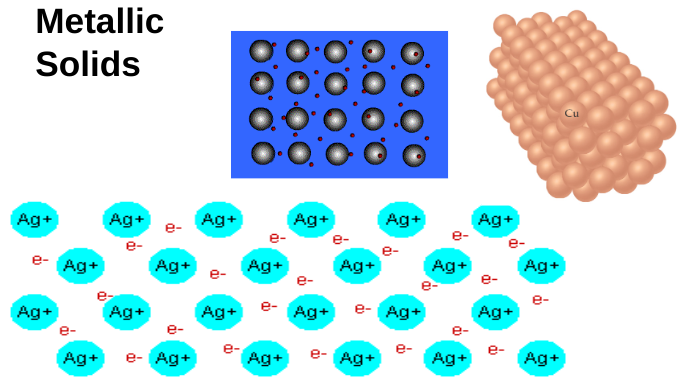
metallic solids
metallic bonding
atoms held together by electrons