Chemistry 3.15- Nuclear Magnetic Resonance Spectroscopy
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
What is NMR?
NMR (nuclear magnetic resonance) spectroscopy is an organic analysis technique that provides information about the positions of atoms in a molecule
1H gives signals based on the positions of hydrogens
13C gives signals based on the positions of carbons
NMR is based on the radio wave frequencies that will cause the spin direction of certain nuclei to flip (measured by changes in its magnetic field), which is dependent on the chemical environments the nuclei are in
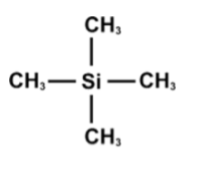
What is the reference compound used for NMR? Why is it suitable?
Tetramethylsilane (TMS) is used to calibrate the spectrum, because:
Non-toxic
Unreactive
Low boiling point, so it is easy to separate from a sample
Produces one strong, sharp peak on the spectrum due to having 12 hydrogens in the same environment
On the chemical shift scale, the TMS signal is always at 0 ppm and the other peaks are measured relative to it
What is important about the solvents selected for use in 1H NMR?
Solvent can’t contain any 1H atoms, as they would show up as signals in the spectrum and hide or disturb other signals
Often solvents containing no hydrogens or containing deuterium (D or 2H, an isotope of hydrogen) are used instead, as they don’t have a nuclear spin and won’t produce signals
eg. CCl4 and CDCl3 (a deuterated solvent)
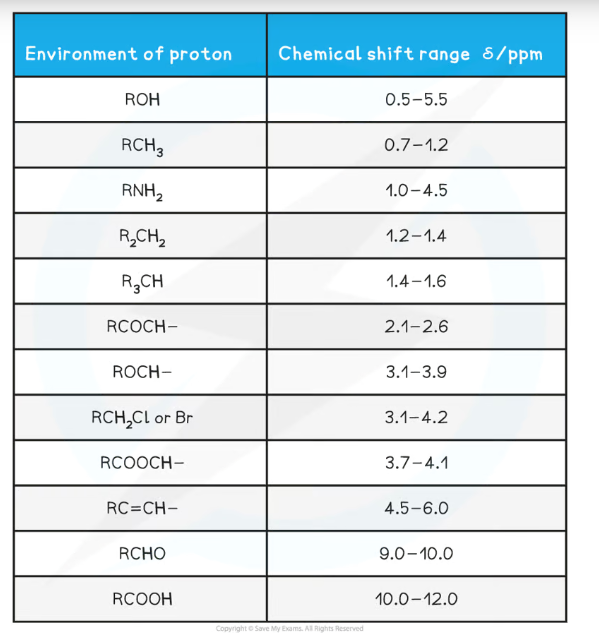
What is chemical shift and how is it used as a scale in NMR?
The chemical shift (δ), measured in parts per million (ppm) is how far away a signal is from the TMS reference signal (which is always placed at 0 ppm)
The closer the environment is to electronegative atoms like oxygen and halogens, the greater the chemical shift
These chemical shift values are interpreted using the data sheet
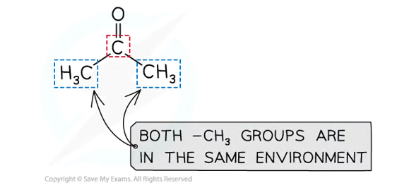
What determines how many peaks an 1H NMR spectrum of a compound will have?
How many chemical environments are present for hydrogens in the compound determines how many peaks are present:
The neighbouring atoms to a carbon influence the field strengths the attached hydrogen will absorb, and so the chemical shift it will show up at
Hydrogens in the same chemical environment are chemically equivalent and will show up in the same peak
Groups that are completely symmetrical to each other are equivalent
eg. 2-methylpropane only has two peaks, because the CH3 groups (red) are equivalent, so those 9 hydrogens all have the same environment- the remaining hydrogen (blue) is in a different environment

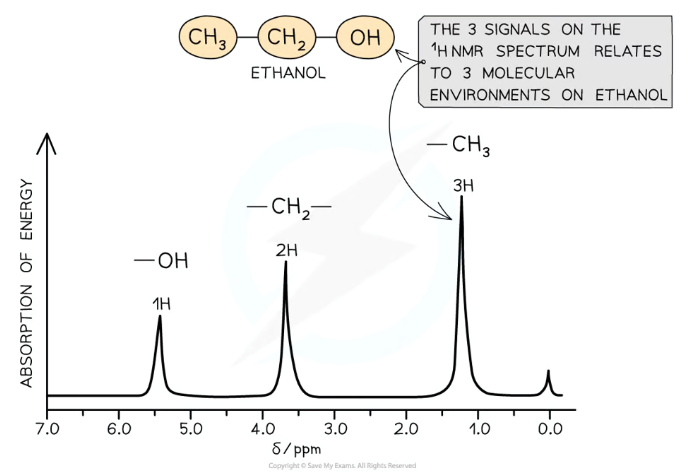
What determines the size of the peaks on an 1H NMR spectrum?
How many hydrogens are present in each chemical environment determines the area of each corresponding peak:
This is measured by integrating the curve to find the area underneath
The areas of the peaks will be in the same ratio as the ratio of hydrogens in each environment
For low-resolution NMR, you can look at the heights to do this, but heights are irrelevant with high-resolution NMR as they have multiple peaks clustered together
eg. There are three peaks for ethanol, with a height ratio of 1:2:3- there’s 1 hydrogen in the -OH environment, 2 in the CH2 environment, and 3 in the CH3 environment
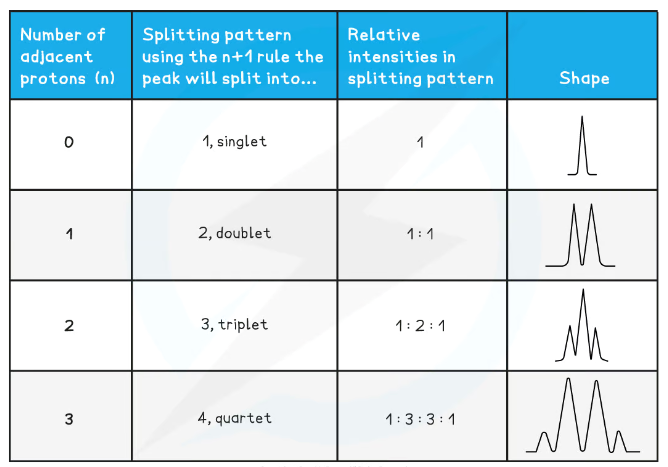
What determines the spin-spin splitting patterns of signals in high-resolution 1H NMR?
The number of hydrogens on atoms neighbouring the environment determines whether signals are split up into smaller clusters (the splitting patterns / coupling / multiplicity):
This follows the n+1 splitting rule- if there are n hydrogens on the atoms adjacent to the environment, the signal will split up into n+1 smaller peaks
An environment with 0 neighbouring hydrogens will only have 1 peak- a singlet
One with 1 neighbouring hydrogen will have 2 peaks- a doublet
One with 2 neighbouring hydrogens will have 3 peaks- a triplet
One with 3 neighbouring hydrogens will have 4 peaks- a quartet
Signals produced by -OH will never split up as the hydrogen isn’t affected by neighbouring hydrogens
How do you interpret 13C NMR spectra?
The number of peaks is equal to the number of distinct carbon environments in a sample, which will each have specific chemical shifts
Unlike 1H NMR, the height and area of peaks are irrelevant and not proportional to the number of carbon atoms in an environment, and there are no complicated splitting patterns
eg. Propanone has two peaks because the CH3 groups are in the same environment, but the C=O is a different environment