Substitution and Elimination
1/17
Earn XP
Description and Tags
SN1, SN2, E1, E2 Review
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
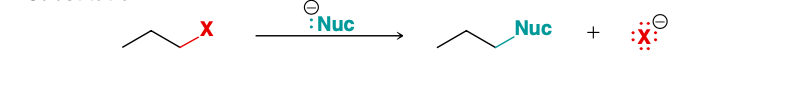
Substitution
Substitution or Elimination RXN?
nucleophile replaces halogen
Elimination
Substitution or Elimination RXN?
Double bond (alkene) is formed
use of a base

bases; acids
Good leaving groups are conjugate ________ of strong _____.
Strong
Strong or weak nucleophiles?
I-
Br-
Cl-
HS-
HO-
N≡C-
RO-
Weak
Strong or weak nucleophiles?
H2O
ROH
Configuration flips
SN2:
if the α position is a chiral center, what happens to the configuration?
Methyl >1° > 2° > 3°
SN2:
Rank the following from most to least reactive:
Methyl, 1°, 2°, 3°
Methyl
In an SN2 reaction, what is most reactive?
Methyl, 1°, 2°, 3°
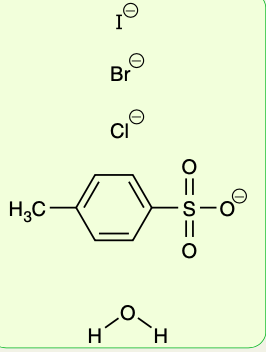
Good leaving groups
Are the following are considered good leaving groups or bad leaving groups?
Recall: Good leaving groups are conjugate bases of strong acids
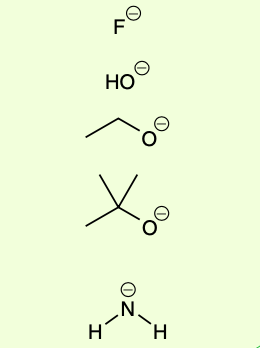
Bad leaving groups
Are the following considered good leaving groups or bad leaving groups?
Recall: Good leaving groups are conjugate bases of strong acids
1,2-elimination
a proton is removed from the β position, the halide leaves from the α position, and a double bond forms between α and β positions
Zaitsev
Zaitsev or Hoffman?
favors the more substituted alkene
Hoffman
Zaitsev or Hoffman?
favors the less substituted alkene
polar aprotic solvents
SN2 favors polar protic or polar aprotic?
Polar aprotic solvents
Protic or aprotic solvents?
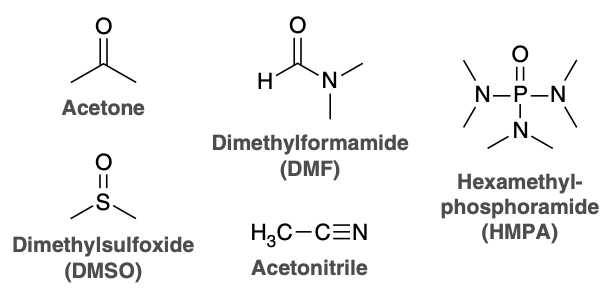
Protic solvents
Protic or aprotic solvents?
Hofmann
What is the major product?
A sterically hindered product
Zaitsev
What is the major product?
A not sterically hindered product