Untitled
0.0(0)
0.0(0)
Card Sorting
1/18
Earn XP
Description and Tags
Last updated 2:13 PM on 9/28/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
1
New cards
Groups
Vertical columns on the periodic table
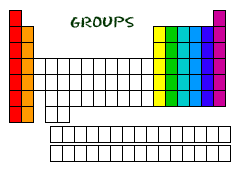
2
New cards
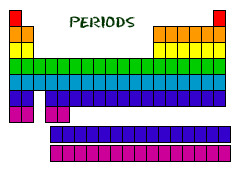
Period
A horizontal row of elements in the periodic table
3
New cards
Metals
an element that is shiny and that conducts heat and electricity well
4
New cards
Nonmetals
Elements that are poor conductors of heat and electric current
5
New cards
Metalloids
Elements that have properties of both metals and nonmetals.
6
New cards
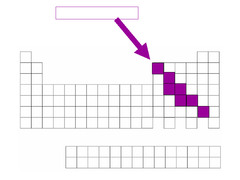
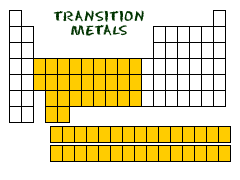
Transition Metals
groups 3-12
7
New cards
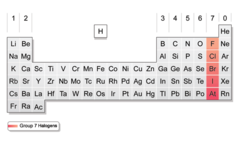
Halogens
Contains nonmetals, 7 valence electrons in it's outermost energy level. Very reactive
8
New cards
Nobel Gases
Atoms that have completely filled energy levels or that eight electrons in their outermost energy level - do not easily lose electrons. ( Group 8 or 18 )

9
New cards
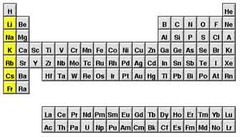
Alkali Metals
any metal in Group 1A of the periodic table
10
New cards
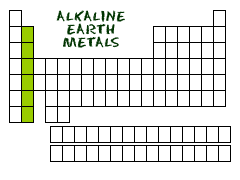
Alkaline Earth Metals
the elements in Group 2A of the periodic table
11
New cards
Atomic Radius
one-half the distance between the nuclei of identical atoms that are bonded together

12
New cards
Ionic Radius
Distance from the center of an ion's nucleus to its outermost electron

13
New cards
Electronegativity
A measure of the ability of an atom in a chemical compound to attract electrons
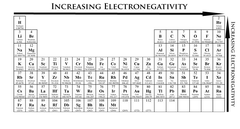
14
New cards
Ionization Energy
The amount of energy required to remove an electron from an atom
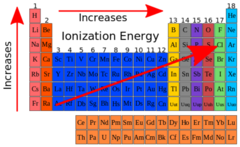
15
New cards
Reactivity
The ease and speed with which an element combines, or reacts, with other elements and compounds.
16
New cards
Cation
A positively charged ion

17
New cards
Anion
A negatively charged ion

18
New cards
Oxidation Number
Positive or negative number that indicates how many electrons an atom has gained, lost, or shared to become stable
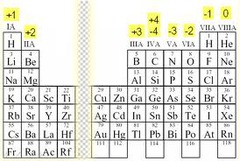
19
New cards
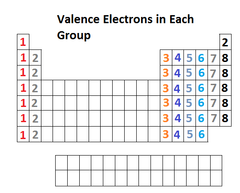
Valence Electrons
Electrons on the outermost energy level of an atom