chem 11 - the atom
1/14
Earn XP
Description and Tags
the historians
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Democritus
first to think about the nature of matter
broke down pieces of matter until he got down to the essence of the matter
came up with the name atom; latin for unbreakable
found out that atoms cannot be created, destroyed or divided
The most powerful people
king
church
both kept “alchemists” who converted metal into gold
Alchemists
coverting metal into gold didn’t work out; instead they invented most techniques and glassware
John Dalton
first came up with an experimentally derived model of an atom
focused on discrete masses of elements (atoms have defined mass) & how they interacted
made the “Billiard Ball Model”: atoms are smallest particle of matter, indivisible sphere with uniform density
stated that different elements combine to form compounds
Sir William Crooks
Cathode Ray Tube (CRT); aka Crooks Tube
a primitive vacuum tube(basis for tube TVs) that generates a continuous flow of electrons to create images
Proved that cathode rays travel in straight lines and carry energy
observed negatively charged particles in gas discharge tubes
JJ Thomson
Proposed the Plum Pudding Model
an atom is a sphere of positive charge with negatively charged electrons (plums) within it
majority of the mass comes from the protons
positive and negative balance each other
PROTONS: dough
ELECTRON: plum
Robert Milikan (milkman)
Put static charges onto oil droplets
the droplets travelled through 2 oppositely charged plates
he measured the smallest increment(change) of charge found in the oil droplets
Discovered the charge of an electron; JJ found out the charge to mass ratio; then he figured out the mass of an electron
Henri Becquerel
Discovered Radioactivity
Discovered X-rays emitting from Uranium Salts
Marie Curie
Discovered the radioactive elements: Radium and Polonium
Ernest Rutherford
worked on Radioactivity
came up with Alpha and Beta radiation
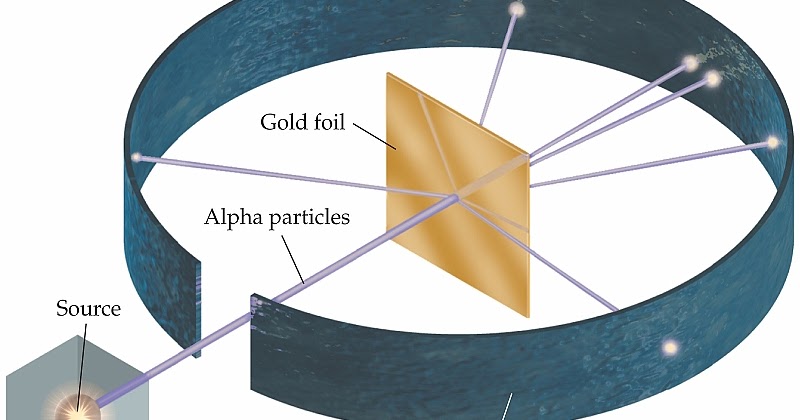
Gold Foil Experiment
shot alpha particles at a thin sheeet of gold foil
“majority” of the alpha particles passed right through the foil
However, some rebounded off the foil
discovered the atomic nucleus, and that atom is made up with PROTONS and NUCLEUS
James Chadwick
Discovered the NEUTRON (inspo of jimmy neutron)
joined the Manhattan Project and participated in developing the atomic bomb.
Niels Bohr
Discovered the Bohr Model
explained the individual spectrum of the elements
electrons exist in specific energy states
energy that was lost is released as protons of light
he assumed that electrons are like planets (circling at a defined distance)
Electrons orbit the nucleus in fixed energy levels (or shells) and can only gain or lose energy by jumping between these levels.
Max Plank
Proposed the "Quantum Theory”
physics of small objects
do not behave like large things
believed that energy was emitted in packets (Quanta) called “photons”
Werner Heisenberg
contributed to Quantum Mechanics (Atomic Theory)
came up with Uncertainty Principle
it is impossible to know both the position and momentum(speed) of a particle at the same time with precision.
Erwin Schrodinger
Contributed to Quantum Mechanics
Described electrons as waves and particles
Stated that electrons do not orbit
Instead there is a cloud where there’s a high “probability” of finding electrons
In an unenergized atom, the electrons are in a “Ground State” (all electrons as close to the nucleus as possible)
Quantum Numbers
used to describe the quantum (energy levels) where electrons might be found; Cat Thought Experiment