Electromagnetic radiation and quantum phenomena
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
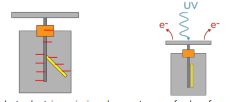
Photoelectric effect
Phenomena of electrons being emitted from a metal surface when UV or higher frequency light is directed at it.
What causes the photoelectric effect?
The light gives electrons the necessary energy to escape the metal.
How does a gold leaf electroscope demonstrate the photoelectric effect?
Gold leaf and stem are negatively charged making them repel each other.
When electrons are emitted from the top plate the repulsion vanishes and the leaf falls.
What suggests that light acts as photons and not waves?
Photoelectric emission does not occur for low frequency light.
If light behaved as a wave only the intensity of the light would affect photoelectric emission.
What did Einstein theorise about photoelectric emission?
Einstein theorised that the energy of a photon is proportional to its frequency (E=hf) so a single electron gains more energy from a higher frequency photon.
Threshold frequency
Minimum frequency required for photoelectric emission to occur.
How does the intensity of the light affect photoelectric emission?
If light is at a high enough frequency for photoelectric emission then its intensity will determine the rate at which electrons are emitted as intensity gives the rate at which photons hit the surface.
Work function of a metal
The minimum energy an electron needs to escape the metal.
Why are electrons emitted with a range of kinetic energies?
As energy required for each electron to escape varies depending on their location.
Which electrons are emitted with the greatest kinetic energies?
Electrons on the surface as energy they use to escape is closest to the work function.
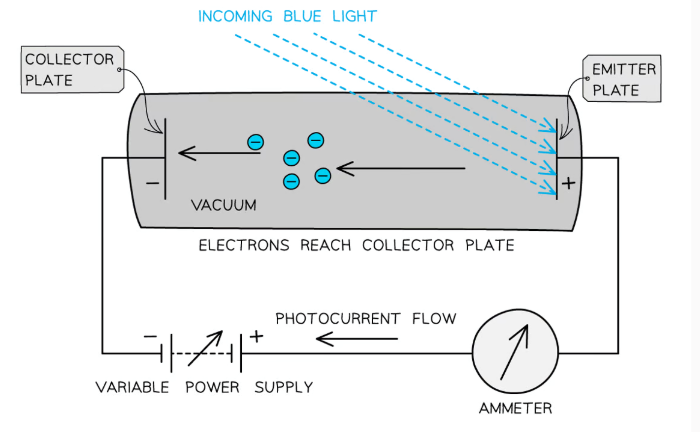
Stopping potential
The potential difference required to stop photoelectric emission from occurring.
It is the potential when applied to the gap between the emitter plate and collecting plate the photoelectric current drops to zero as no electrons have enough energy to cross the gap.
Electron volt
Work needed to move an electron through a potential difference of 1V.
Explain how a fluorescent tube works
Fluorescent tubes contain mercury at low pressure, with filament electrodes at both ends and a fluorescent coating on the inner surfaces.
Electrons accelerate between the electrodes, ionising the mercury to produce more free electrons and exciting mercury electrons which de-excite to emit UV photons which are absorbed by the fluorescent coating.
The atoms in the coating become excited and de-excite in multiple steps to produce many visible light photons.
How do electrons exist in atoms?
Specific energy levels
Ground state
Energy level n=1 which is the lowest energy level
How can the energy needed to be supplied to an electron to escape the atom be found?
The energy of the level the electron is located id the energy required for it to escape the atom.
Ionisation energy
Energy needed to remove an electron from the ground state.
What is the energy of a photon emitted during de-excitation equal to?
The difference between the energy levels the electron moves between hf=ΔE=E2-E1.
Emission spectrum
Produced when passing photons emitted from an excited gas through a prism.
Only certain discrete lines of colour will be on the spectrum corresponding to the frequencies of photons with energies equal to the possible changes in energy levels in the gas.
This is because these photons are produced by de-excitation of electrons.
Absorption spectrum
Produced by passing white light through a cool gas before a prism.
The spectrum will be mostly continuous with discrete lines of missing frequencies which correspond to the frequencies of photons with energies equal to the possible changes in energy levels in the gas.
This is because these photons can be absorbed by electrons in the gas to excite them.
How do emission and absorption spectra provide evidence for existence of energy levels?
Lines are discrete meaning that only specific frequencies of light are absorbed and emitted by atoms in a gas.
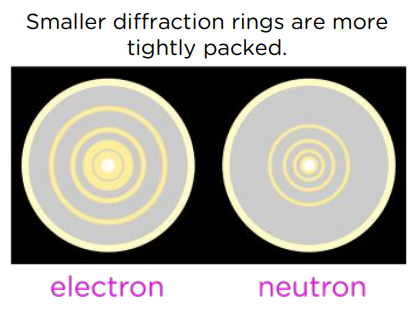
What happens when accelerated electrons pass through spaces in a graphite crystal?
Electrons diffract through the gaps producing a diffraction pattern of concentric rings.
What does electron diffraction tell us?
Particles possess wave like properties.
How does increasing the accelerating voltage affect the diffraction pattern?
Electrons gain more kinetic energy so have a smaller de Broglie wavelength.
This causes less diffraction and results in smaller diffraction rings more tightly packed.
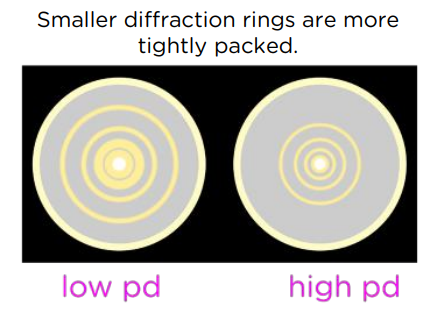
How does using particles with greater mass but with the same speed affect the diffraction pattern?
Particles have more momentum due to their higher mass so have a smaller de Broglie wavelength.
This causes less diffraction and results in smaller diffraction rings more tightly packed.