AC6 - UV-vis spectroscopy
1/19
Earn XP
Description and Tags
understand the basic principle of a wave ,explain absorption by matter and describe beer-lambert law
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
How does light split ?
white light split into many components
naturally we only see what is visible to us but there is a lot more light than we see
What can light be described as ?
light can be described as a particle
Describe the components of a wave ?
wavelength ( lambda) - the distance between 2 equivalent points in successive cycles , measured in cm ,micrometer or nm
Amplitude - the maximum value reached by the wave in a given direction over a cycle
Frequency - the number of wavelengths passing a fixed point in one second
( f)
velocity - speed of the wave
What is the equation for frequency ?
Thus, for any wave:
f= V/𝛌
As we are dealing with light with has a velocity of 3 x 108 m s-1 which has the symbol c we replace v to give us:
f= c/𝛌
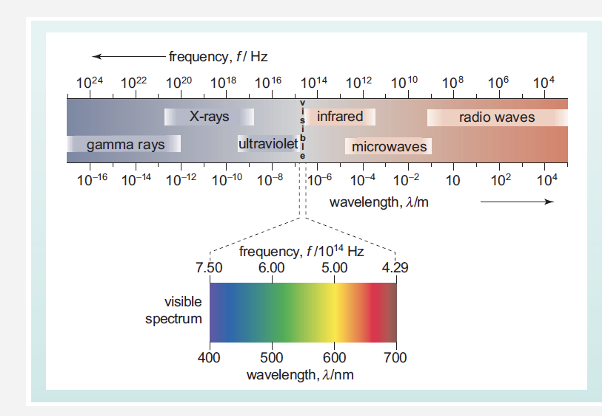
What is the electromagnetic spectrum ?
Matter absorbs light differently depending on the strength of the light
Molecules and atoms absorb energy. This energy relates to various regions of the electromagnetic spectrum (right).
This absorption is dependent on the chemical nature of the molecule or atoms
Em spectrum can be used to identify and confirm the nature of a chemical compound
Describe light as a particle?
Einstein proposed that light of any specific colour consists of identical particles , each with a particular energy depending on the colour of the light
These particles of light are called photons
The amount of energy carried by a single photon is called quantum
This gava an alternative simple model of light - that was very different from EM wave model. this theorizes each specific wavelength has a corresponding “particle”
Describe the alternative simple model ?
According to this model , a ray of light of frequency f can be thought of as a stream of separate particles called photons
Each photon carries identical energy ( E) ,which relates directly to the frequency 🇦 :
E=hf
h is plancks constant
How is energy absorbed ?
energy absorbed in packets - Photons
the energy of a photon is directly proportional to the frequency of the light ( EM Wave ) EQUATION 1 : E= hf
as frequency can be calculated by EQUATION 2 : f= c/𝛌
we can replace f in eq 1 to obtain EQUATION 3 : E= hc/λ
Eq 3 shows that the energy of a photon is inversely proportional to wavelength
as wavelength of light increases the energy of the photon will decrease
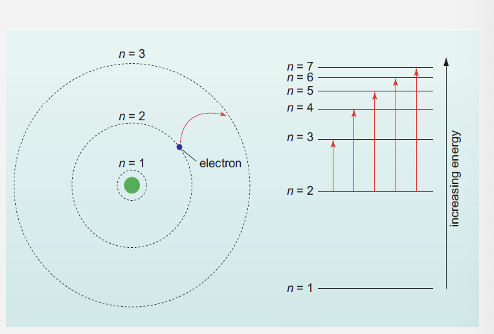
What happens for absorption for uv-vis region ?
the uv-vis photons are absorbed by atoms
this absorption causes an electronic transition - causing an electron to jump an energy level
the energy required to move an electron from n=2 to n=3 is less than to make it move from n=2 ,n=7
only a photon of a specific energy corresponding to the energy gap between energy levels of that transition will be absorbed by the electron
What are the electronic transitions observed in uv-vis spectroscopy ?
The electronic transitions primarily observed in UV-Vis spectroscopy are:
π → π*
n → π *
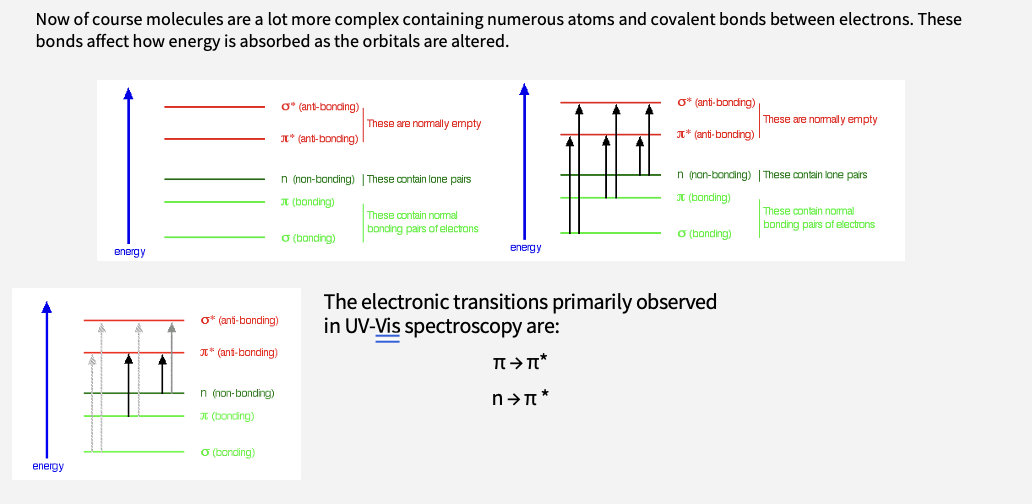
What happens if you have broad absorption ?
The broad absorption spectra occurs as all of these combine to absorb light throughout the uv-vis portion of the ems
certain molecular groups exhibit certain absorption patterns
these ar ecalled chromophores - if they are in the visible range- coulured
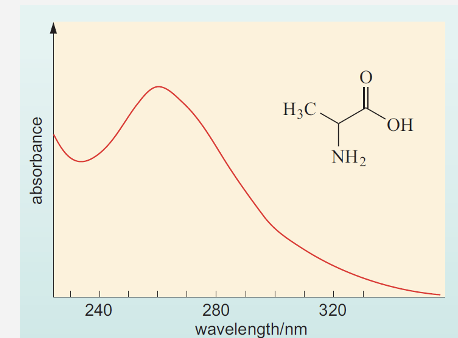
How do we use spectras ?
This spectra is of alanine, a fairly simple molecule but still shows broad absorption.
For our ease we can use the position of highest absorption as a reference point for measurement.
We refer to this as lambda max (λmax). – use when measuring a compound
Here this would be 262 nm.
By using λmax we have a starting point to detect our compound.
Therefore, if we use a spectrophotometer to measure absorption at 262 nm we could detect the presence of alanine in a solution.
How is light absorption by a sample determined using a sample cell and a reference cell in spectrophotometry?
Incident light: I0I_0I0
Sample cell (sample + solvent): measured light I1=I0−(S+R)
Reference cell (solvent only): measured light I2=I0−RI
The light transmitted by the sample alone is:
I=I0−(I2−I1)
If I1<I2 , the sample has absorbed light at that wavelength.
This absorption is recorded as absorbance (A) and plotted versus wavelength to form a spectrum.
What is a monochromator?
●monochromator- changes white light to a specific wavelength
What is beer-lambert law ?
The Beer–Lambert law states that absorbance is directly proportional to the concentration.
How can intensity of light be converted to absorbance ?
A = log10(I0/I)
Where:
•I0 is the intensity of light that is incident on the sample;
•I is the intensity of light that is transmitted by the sample only.
what equation links absorbance and concentration through beer-lambert law ?
A = εcl
where:
•c is concentration (mol l−1),
•l is the pathlength of the cell(cm),
•ε is a proportionality constant called the molar extinction coefficient
What is the molar extinction coefficient ?
The molar extinction coefficient is a measurement of how strongly a chemical species absorbs light at a given wavelength; its unit is litres per mole per centimetre (l mol−1 cm−1).
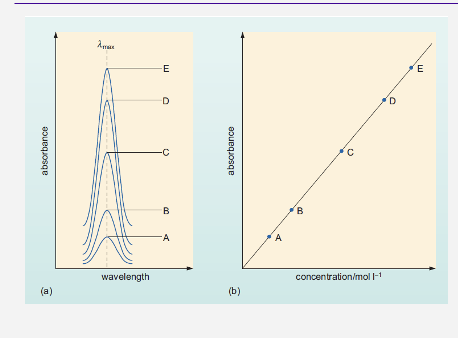
Describe The beer-lambert law graph ?
•If we take a series of diluted samples we can use UV-Vis to measure them.
•As ε and l will stay the same.
•The former related to the molecule and the latter based on the cuvette…
…so we can plot A against c directly
•In this plot, the gradient is ε.•
•This will give us a calibration curve, from which we can calculate any concentration in range.
What are the 5 conditions which allow a linear relationship for beer- lambert law?
1.The absorbance at the chosen wavelength is generated by one species in the sample. – pure sample ( if something else absorbs can’t relate the beer lambert law )
2.The absorbing matter must be distributed evenly in the solution in the cuvette and must not scatter the radiation. This means that solutions with suspended particles(undissolved material) cannot be measured accurately.
3.The light must travel in the same direction through the sample solution.
4.The light should preferably be single wavelength, to ensure there is no effect from other radiation wavelengths.
5.The radiation must not influence the atoms or molecules in any other way, other than absorbing in the region of interest. - because it may cause different types of absorbance