Design Strategies and Statistical Methods in Epidemiology: Causality, Bias, and Study Types
1/175
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
176 Terms
What is analytic epidemiology?
A study that attempts to answer why and how a health-related state or event occurred, testing specific a priori hypotheses.
What are the two main types of analytic epidemiologic studies?
Observational and experimental studies.
What is the purpose of a case-control study?
To group people as cases or controls and evaluate the likelihood of a health event in the case group compared to the controls.
What does 'retro spicere' mean in the context of case-control studies?
It means 'to look back', indicating that the study starts with the outcome and looks back at antecedent variables.
What is the first step in conducting a case-control study?
Establishing the diagnostic criteria and definition of the disease.
How can cases be selected for a case-control study?
Cases can be new (incidence) or existing (prevalence) and should be representative of all persons with the disease.
What is the significance of random selection in case studies?
Random selection ensures that the cases are representative and improves the validity of the study.
What is the role of exposure status in case-control studies?
Exposure status must be ascertained to evaluate the relationship between exposure and outcome variables.
What are common methods to ascertain exposure status?
Medical records, interviews, questionnaires, and surrogates.
What is the odds ratio (OR) in epidemiology?
A measure of association used in case-control studies that compares the odds of disease among exposed individuals to unexposed individuals.
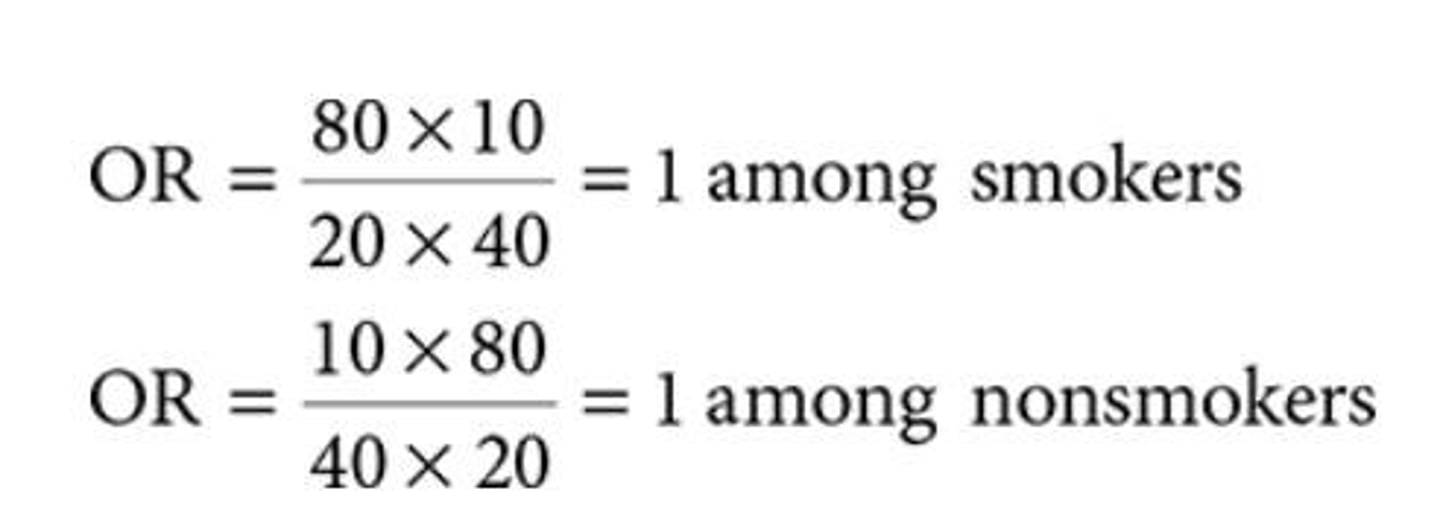
What does an odds ratio of 1 indicate?
No association between exposure and disease.
What does an odds ratio greater than 1 indicate?
A positive association between exposure and disease.
What does an odds ratio less than 1 indicate?
A negative association between exposure and disease.
What is bias in case-control studies?
Systematic error in the collection or interpretation of epidemiologic data, leading to inaccurate estimations of association.
What is selection bias?
Bias that occurs when cases and controls are selected based on exposure, affecting the relationship between exposure and disease.
What is Berkson's bias?
A type of selection bias in hospital-based case-control studies where controls may be in higher risk categories.
What is prevalence-incidence bias?
A form of selection bias attributed to selective survival among prevalent cases, leading to inaccurate estimations.
What is the importance of blinding in case-control studies?
Blinding prevents bias by ensuring that interviewers or record assessors do not know the case vs. control status.
What are the two types of observational studies?
Exploratory (no specific hypothesis) and analytic (specific a priori hypothesis).
What is the function of a 2 x 2 contingency table in epidemiology?
To summarize the relationship between exposure and health outcome variables.
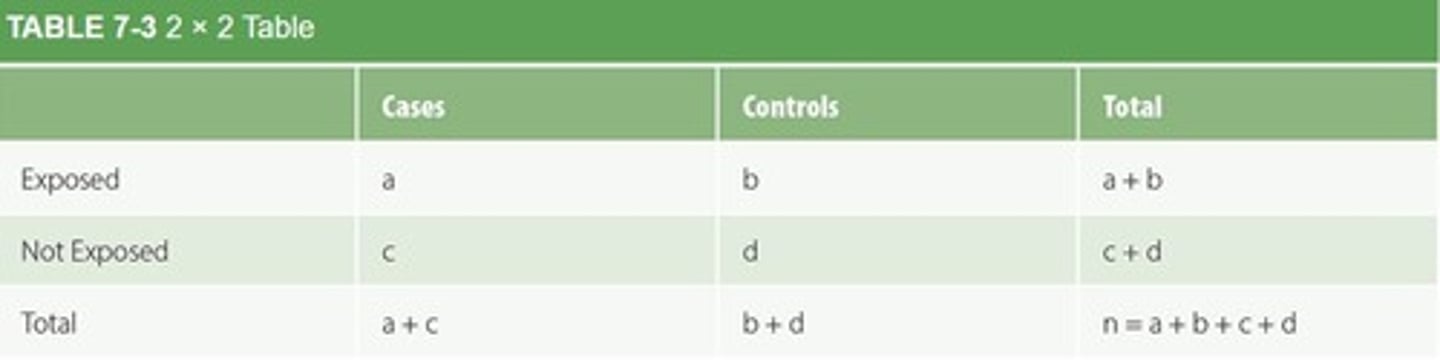
What is the primary goal of an epidemiologist conducting a study?
To conduct a valid study with definitive results.
What is confounding in epidemiology?
A situation where a third variable influences both the exposure and outcome, potentially distorting the perceived association.
What is effect modification?
When the effect of the main exposure on an outcome differs depending on the level of another variable.
What are the two main forms of observation bias?
Recall bias and interviewer bias.
What is recall bias?
Differential accuracy of recall between cases and controls, where cases may remember exposure status better due to their situation.
Give an example of recall bias.
A woman with a child who has neurological problems may better recall having the flu and high temperature during pregnancy.
What is interviewer bias?
When an interviewer probes cases differently than controls, potentially influencing the responses.
How can observational bias be avoided?
By using medical records instead of self-reported information.
What is misclassification in epidemiological studies?
It occurs when either exposure or outcome status is inaccurately assigned.
What is random misclassification?
Equal misclassification of both cases and controls, which occurs at some level in all case-control studies.
What is nonrandom misclassification?
Unequal misclassification that occurs among groups, often resulting from selection and observation bias.
What is confounding?
When an extrinsic factor is associated with an outcome and is also linked with an exposure.
What are routine confounders?
Common confounders include age, sex, educational level, and smoking.
What is a case-crossover study design?
It compares the exposure status of a case immediately before its occurrence with that of the same case at a prior time.
In what context are case-crossover studies increasingly common?
In environmental epidemiology, particularly for intermittent exposures.
What is a nested case-control study?
A case-control study that is 'nested' within a cohort study, comparing exposure status of selected cases and noncases.

Provide an example of a nested case-control study.
Study of French uranium miners who died of lung cancer, comparing 100 miners with 500 matched controls.
What does a cohort study refer to in epidemiology?
A group of persons being studied who were born in the same year or time period.
What is the difference between prospective and retrospective cohort studies?
Prospective studies measure predictor variables before outcomes occur, while retrospective studies reconstruct historical cohorts with past data.
What is a cohort effect?
Changes in disease or health status of a study population as the group moves through time, influenced by shared exposures.
What is the risk ratio in cohort studies?
A measure of association reflecting the probability of a health-related event among those exposed relative to those not exposed.
What is a rate ratio?
A measure of association used when person-time units are available, comparing rates of events between groups.
What is a double cohort study?
A study involving two distinct populations with different levels of exposure to an interest, often used for rare exposures.
What is a significant risk associated with mine work in the context of a nested case-control study?
Significant risk of lung cancer associated with mine work after adjusting for smoking.
What are the strengths of case-control studies?
Useful for studying rare outcomes, short duration, relatively inexpensive, and yields odds ratios.
What are the weaknesses of case-control studies?
Does not establish sequence of events, potential bias in measuring exposure variables, and limited to a single outcome variable.
What is the significance of using odds ratios in case-control studies?
Odds ratios are usually a good approximation of relative risk.
What is the purpose of selecting a study cohort?
To choose individuals at risk of becoming a case while excluding those who already have the disease or are not at risk.
What is selection bias in cohort studies?
A distortion that occurs when the participants selected for a study are not representative of the general population.
What is the 'healthy worker effect'?
A phenomenon where working populations are generally healthier than the general population, leading to biased results.
What is loss to follow-up in cohort studies?
When researchers lose contact with study participants, resulting in unavailable outcome data, which can compromise study validity.
What is the maximum acceptable percentage of loss to follow-up in a study?
Loss to follow-up should not exceed 20% to maintain the validity of a study.
What is confounding in epidemiological studies?
An outside influence that distorts the perceived association between an exposure and an outcome variable.
How can misclassification occur in cohort studies?
It can happen when individuals are incorrectly categorized regarding their exposure or outcome status.
What strategies can minimize loss to follow-up bias?
Restricting participants to those likely to remain in the study, collecting personal identifying information, and providing incentives.
What is the role of randomization in experimental studies?
To assign participants randomly to different intervention groups to eliminate bias and ensure comparability.
What is the purpose of blinding in experimental studies?
To prevent bias by keeping study participants and/or researchers unaware of which intervention is being administered.
What are the two main types of experimental studies?
Clinical trials and community trials.
What is a clinical trial?
A controlled study that evaluates the efficacy and safety of a new drug or medical procedure on human subjects.
What is a prophylactic trial?
A type of clinical trial used to evaluate preventative measures, such as vaccines or contraceptives.
What defines a community trial?
A study that tests a group intervention aimed at educational and behavioral changes at the population level.
What is a natural experiment in epidemiology?
An unplanned event that creates conditions resembling a controlled experiment, allowing for the study of exposure effects.
What is the unit of measurement in a clinical trial?
The individual participant.
What is the unit of measurement in a community trial?
The group receiving the intervention.
What is the strength of a community trial?
It controls for individual confounding factors.
What is a weakness of community trials?
They are susceptible to time-related factors that may affect outcomes.
What is the purpose of stratification in analysis of cohort studies?
To adjust for potential confounders by analyzing subgroups separately.
What is a factorial design in experimental studies?
A design that evaluates multiple interventions simultaneously to understand their effects and interactions.
What ethical issues are associated with experimental studies?
Concerns about informed consent, potential harm to participants, and the fairness of treatment allocation.
How can researchers control for confounding at the study design level?
By restricting participant selection to avoid bias and ensuring comparison groups are as similar as possible.
What is the importance of refining definitions of exposed and unexposed groups?
To minimize misclassification and ensure accurate outcome ascertainment.
What were the ten-year cumulative incidences of radical prostatectomy and external beam radiation in Seattle?
2.7% and 3.9%
What were the ten-year cumulative incidences of radical prostatectomy and external beam radiation in Connecticut?
0.5% and 3.1%
What was the adjusted rate ratio of prostate cancer mortality between Seattle and Connecticut from 1987 to 1997?
1.03 (0.95 to 1.11), indicating no significant difference.
What is the purpose of randomization in clinical trials?
To make intervention and control groups as similar as possible, minimizing confounding.
What is a randomized controlled trial?
The most common type of trial in clinical settings where participants are randomly assigned to groups.
What is the significance of blinding in experimental studies?
To minimize potential bias from the placebo effect.
What is a placebo?
A substance containing no medication or treatment given to satisfy a patient's expectation of getting well.
What are the three levels of blinding in studies?
Single-blind, double-blind, and triple-blind.
What characterizes a single-blind study?
Subjects are blinded, but investigators know who receives the treatment.
What characterizes a double-blind study?
Neither the subjects nor the investigators know who receives the active treatment.
What characterizes a triple-blind study?
Subjects, investigators, and analysts are all blinded to treatment assignments.
What are some advantages of randomized controlled trials?
Eliminates conscious bias, averages out unconscious bias, and groups are alike on average.
What are some disadvantages of randomized controlled trials?
Ethical issues and interference with the doctor-patient relationship.
What are some reasons randomization may not be possible?
Insufficient population size, funding issues, or lack of participants with the disease.
What is a concurrent comparison group?
A group allocated by a nonrandom process when randomization is not feasible.
What is the role of a protocol in designing a clinical trial?
It is a detailed written plan outlining the study's research questions, design, and statistical issues.
What are the six steps involved in designing a randomized controlled trial?
1. Selecting an intervention, 2. Assembling a study cohort, 3. Measuring baseline variables, 4. Choosing a comparison group, 5. Ensuring compliance, 6. Selecting the outcome.
What is the purpose of Phase I trials?
To determine the safety of a test in humans, usually involving fewer than 30 patients.
What is the typical patient profile for Phase I trials?
Patients often have advanced disease and have already tried other treatment options.
What ethical considerations should be taken into account during trial design?
Ensuring the safety and rights of participants throughout the study.
What is the significance of ensuring compliance in clinical trials?
To maintain the integrity of the study and ensure accurate results.
What is one potential problem with nonrandomized studies?
They may not effectively control for unmeasured confounding variables.
How can blinding help avoid unconscious bias in clinical trials?
By ensuring that physicians and outcome assessors do not know which treatment participants receive.
What is a common challenge in drug studies regarding blinding?
If the treatment has characteristic side effects, it may be unethical to induce those in the placebo group.
What characterizes patients in phase I clinical trials?
Patients often have advanced disease, have tried other options, and undergo intense monitoring.
What is the purpose of phase II clinical trials?
To test tolerability, safe dosage, side effects, and how the body copes with the drug, as well as to evaluate effectiveness against specific diseases.
What is the typical size of phase II clinical trials?
Relatively small, involving up to 50 people.
What happens in phase III clinical trials?
They involve larger groups, often thousands of patients, to evaluate the efficacy of a new treatment through random assignment.
What is the outcome of a successful phase III trial?
It may lead to FDA approval of the treatment.