NEUR201 Module 1 (neurophysiology)
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
Nerve cells, CNS, PNS
neurons & glial cells in both CNS and PNS
Brain, spinal cord
peripheral nerves & ganglia, axons can be unmyelinated
Neuron structure
Input zone= dendrites & cell body, receives chemical signals from other neurons
Summation zone= axon hillock, summation of inputs
Conduction zone= Axon, carries electrical signals
Output zone= axon terminals, contact with input zone of other neurons or effectors, release of neurotransmitter= chemical signal
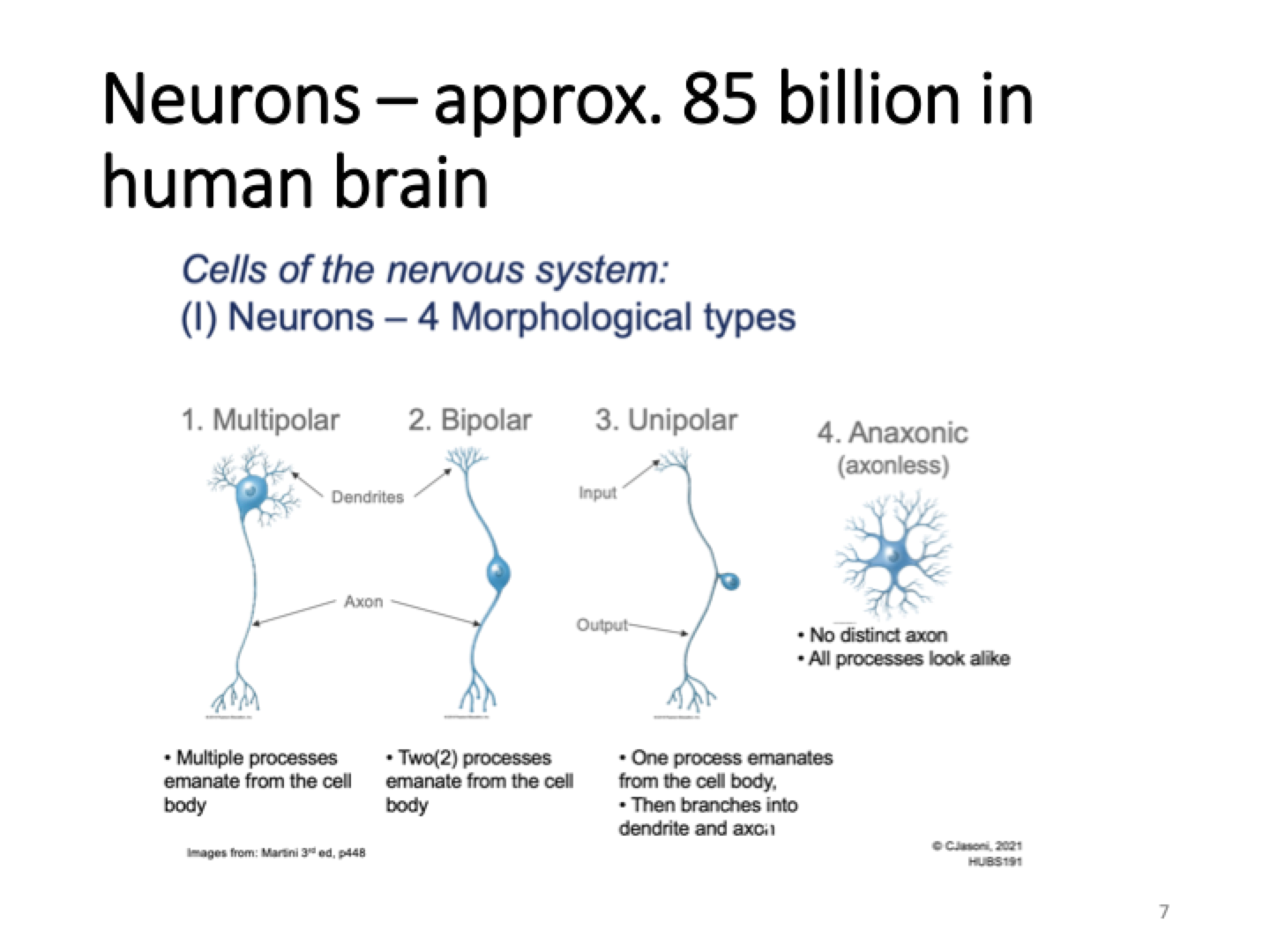
Types of neurons
multipolar= multiple processes emirate from cell body
Bipolar= 2 processes eminate from cell body
Unipolar= axon hillock is right below dendrites
Anaxonic= no distinct axon
AP is generated at the base of axon and conducted along axon.
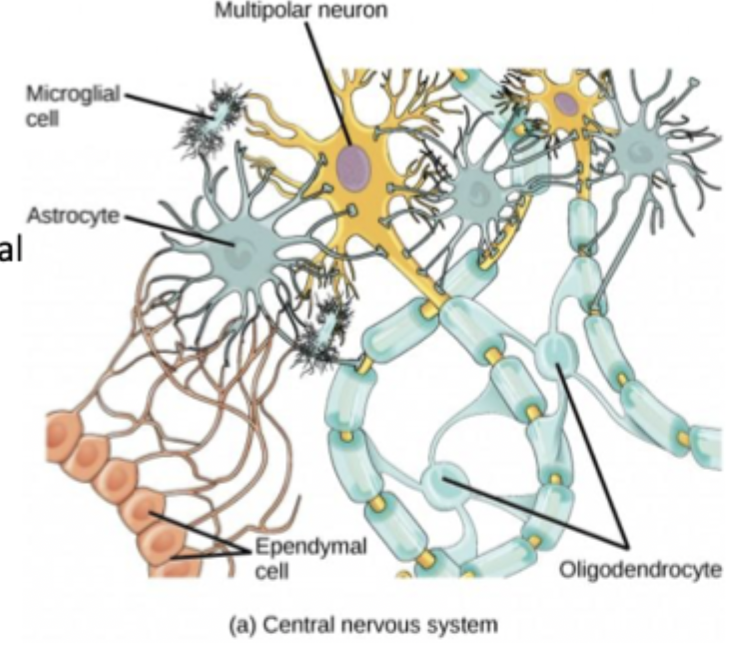
Glia in CNS
Oligodendrocytes form myelin sheath, each oligodendrocyte can provide myelin for more than one axon in the CNS
Astrocytes provide nutrients, maintain extracellular environment and provide structural support
Microglia immune response
Ependymal cells circulate and produce cerebrospinal fluid
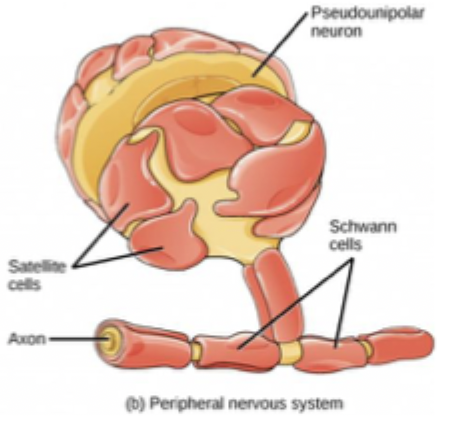
Glia in PNS
50 billion glia in human brain
Shwann cells form myelin sheath
Satellite cells provide nutrients and structural support to neurons
where electrical signal becomes chemical
If AP is generated it is conducted along axon and delivered as output (at input of next neuron), at the nerve terminal electrical signal turns into chemical signal
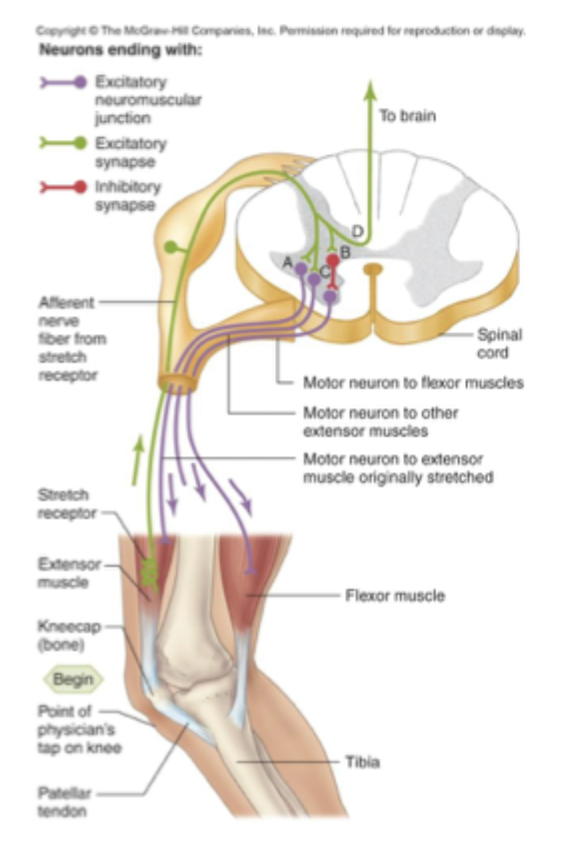
Stretch reflex network
At peripheral nerve
Peripheral sensory receptor senses muscle stretch bc it has ion channels
if muscle is stretched APs are generated and conducted to output zone which is in the PeripheralNS
cell in the PNS is brought to threshold= efferent axon sends signal to muscle= muscle contracts
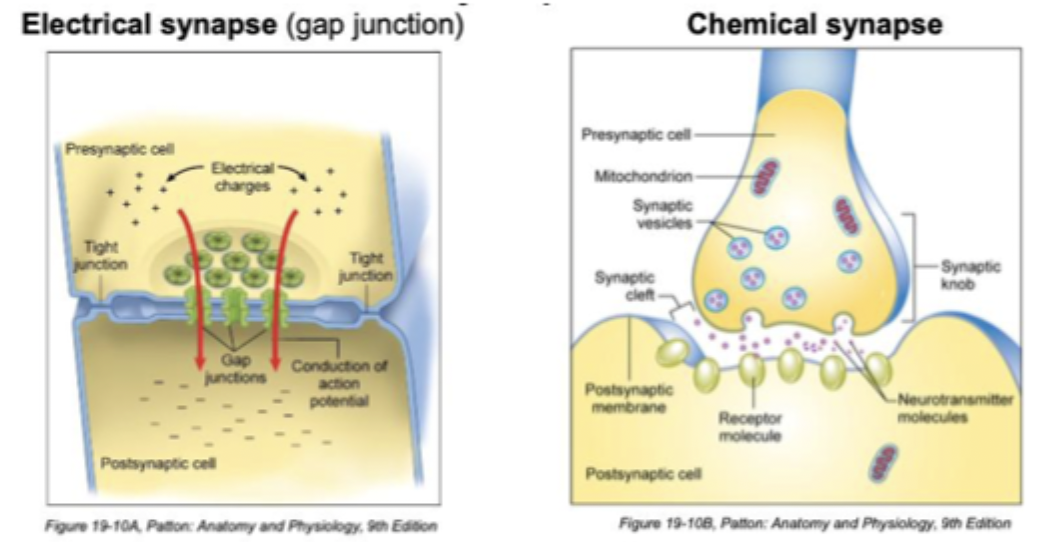
Electrical synapse vs Chemical synapse
Very fast, ions flow from cell to cell, may be bidirectional
Slower, relies on chemical crossing gap (synaptic cleft), neurotransmitter packaged in vesicles, only unidirectional
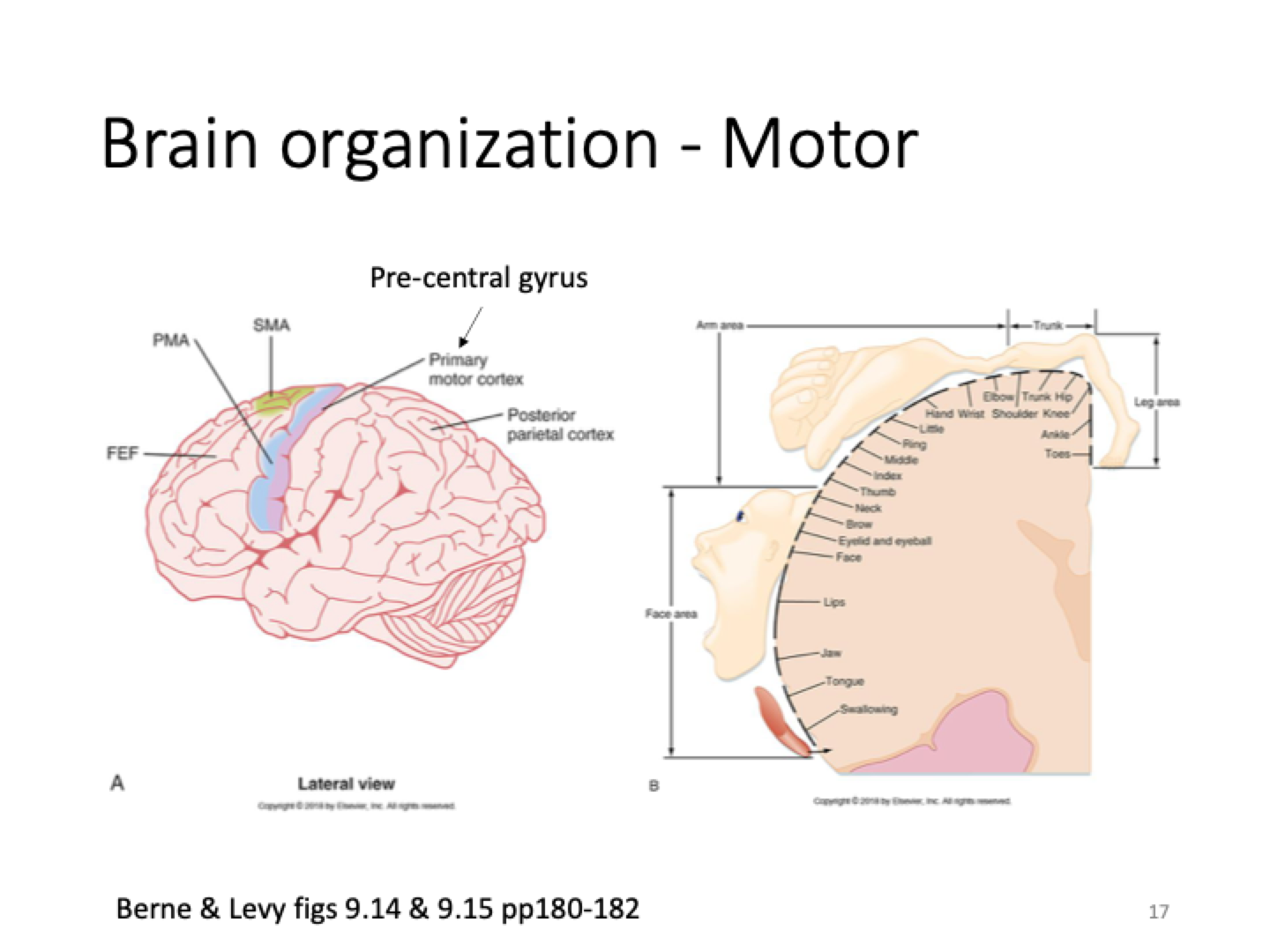
Motor brain organisation
Large number of nerve cells dedicated to movement in mouth, lips
Trunk and hips have less nerve cells for movement so move less
Sensory Efferent vs Motor Affarent info flow
Info from receptors, to spinal nerves, brain & spinal cord
Somatic= skeletal muscle effector, autonomic= sympathetic (NE, fight or flight), parasympathetic (ACh, rest & digest)
Resting membrane potential, local/action potentials, signalling between nerve cells or non-nerve cells (e.g. muscles)
electrical potential across membrane of inactive nerve cell
when excitable cells are active, the potential (voltage) across the membrane briefly changes
mostly chemical
ICF, ECF, cell membrane separating them
Low Na+, high K+
high Na+, low K+
Regulates exchange of substances between cell and environment, stops ICF & ECF from mixing
Cell/plasma membrane
barrier to free movements of ions
made of lipids (hydrophobic) and proteins
water can’t freely cross the hydrophobic membrane
Steroid hormones (e.g. testosterone) are lipid soluble (non-polar) so can freely cross membrane
Transport proteins (channel/carrier), leak channels, gated channels
facilitate ion movement across the plasma membrane which allows for controlled movement
open ion channels
ion channels opened in response to stimulus
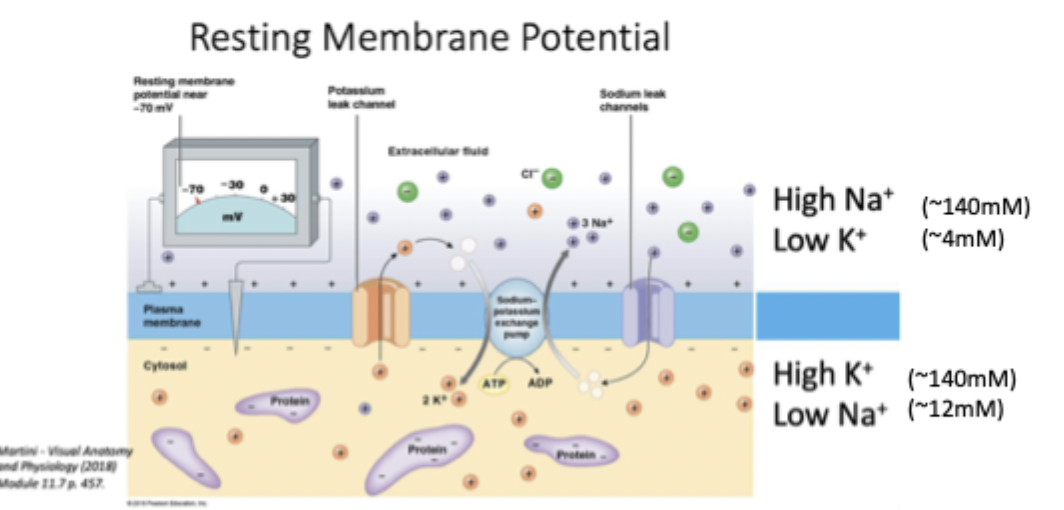
Ion gradients for nervous system function (RMP, NA/K ATPase, potassium leak channel)
Inside of the cell is -70mV relative to outside
Brings K+ in and Na+ out using ATP (bc low to high conc) to maintain gradient
Protein selectively allows K+ to go out of the cell (high to low so no ATP). This is important for maintaining RMP of -70.
Electrochemical gradients (chemical gradient, electrical gradient) at RMP (-70mV)
High to low conc. Na+ in, K+ out
Opposite charges attract. Na+ in, K+ in because charges are positive and the cell is negative .
Ion channels, 3 properties
Open to ECF and ICF at the same time
Selectivity of what passes through
Conductance (how many pass through)
gating (signal controls when it is open or closed, external influences: mechanical, chemical, electrical)
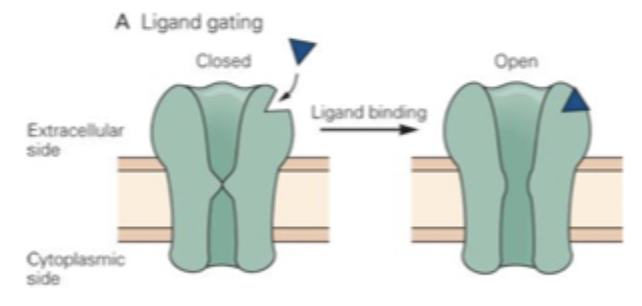
Chemically/ligand gating
Ligand binds to binding site
Bound channel changes configuration, and pore appears
Ligand unbinds and channel returns to original (closed) configuration
E.g. nAChR binds nicotine
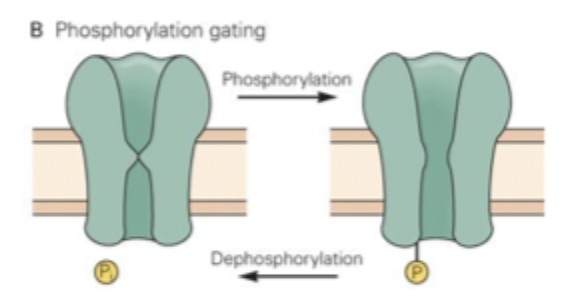
Phosphorylation gating
Activation of second messenger pathways (often by neurotransmitter interactions with GPCR)
Can result in ion channel opening via phosphorylation
e.g. mAChR
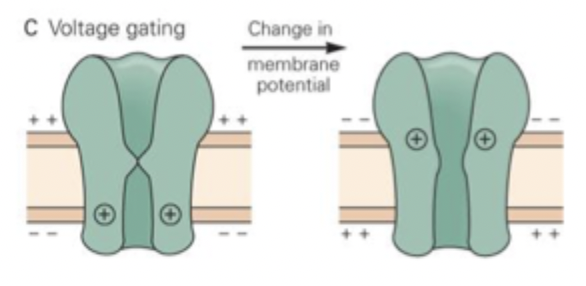
Voltage gating
change in MP changes channel configutation
has a charged region (voltage sensor) that triggers a change in shape when the voltage across it changes
some VG channels can be inactivated (refractory) after opening. Reactivated by MP being restored so they can open again
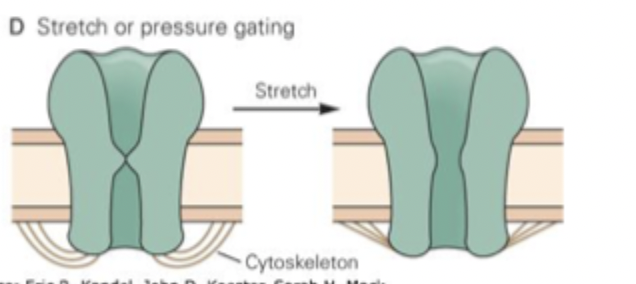
Mechanical gating
opened by mechanical force (e.g. stretch, pressure) applied to the membrane
membrane permeability increased in response to physical force
Important for touch, hearing, balance, bodily functions
Transporters (carrier proteins)
cotransporters, exchangers, pumps
carrier protein Binds solute and transports it across the membrane
low rates of solute transport
Not open to ICF and ECF at the same time
Active transport transporters, primary, secondary
moves substances against conc gradient
Uses energy (e.g. ATP)
Uses electrochemical gradient (symporters move one down and one up gradient in the same direction e.g. Na-glucose, Antiporter does the same but in opposite directions e.g.Na-Ca)
Transporters (passive transport)
Facilitated diffusion= down conc gradient
Limited speed of changes
resting nerve cell permeability (chemical gradient), electrostatic force
large number of K+ leak channels means resting nerve cell is 100x permeable to K than Na. So if Na conc changes RMP barely changes.
Strong chemical gradient drives K+ out of the cell through channels which leaves a slight positive deficit in the cell making the cell slightly negative inside and outside positive
since K+ is moving out of the cell in chemical gradient the voltage difference across membrane increases so electrostatic force increases
equilibrium potential (Ek), RMP
log10 x (out/in)
transmembrane voltage where outward chemical movement of K+ down gradient matches inward K+ electrical movement up gradient
so resting membrane potential is close to Ek but Ena makes the cell slightly more negative
K, Na and Cl have an impact on membrane potential
K diffuses out of the cell generating negative charge inside, it will go out until negative reaches -94mV where the electrical charge balances outward diffusion
If membrane was only permeable to Na then membrane potential would be +65mV
Combining K and Na, Ek= -86mV
RMP when at rest, when cell is only permeable to Na
Steady state= No net loss or gain of K because electrochemical gradients are balanced, no net loss or gain of Na at rest the cell is relatively impermeable to Na
Na enters cell down electrochemical gradient, more positive charge in cell, cell more positive. until +65mV but this would only happen if the channel stayed open (rare)
Ion channel event results in increase in Na conductance, local current
Cells Na permeability increases, electrochemical gradients favour Na+ entry, positive charge into cell, depolarisation (excitatory local potential)
Neurotransmitter released into synaptic cleft and binds to receptor, Na+ goes into cell, cell inside less negative/more positive
Local current= electrical current (voltage) spreads a bit sideways which spreads the positive Na slightly. This is depolarization because the cell became less negative. The cell is now more likely to release an action potential.
Increase in K+ conductance
RMP is more positive than the potassium equilibrium potential (Ek)
Increasing the cells conductance to K+ will cause K+ to flow out of the cell (down electrochemical gradient) which carries positive charge with it to make the cell more negative. This is to drive the cell towards the Potassium equilibrium potential (Ek).
If neurotransmitter was released and bound to neurotransmitter gated potassium channel, if cells membrane potential is more positive than the cells equilibrium potential the membrane potential will become more negative.
Hyper polarization (inhibitory/IPSP) would occur since the membrane potential is more negative
Local potential
Inhibitory or Excitatory
Likelihood of cell being sufficiently depolarized enough to reach threshold depends on whether the excitatory input is bigger than the inhibitory input. If inhibition is bigger than excitation, the excitation won't do anything.
Local potential vs AP
Graded, decremental, may not reach threshold, in dendrites/cell body
All or none, self-propagating, much reach threshold to fire, in axon hillock → axon
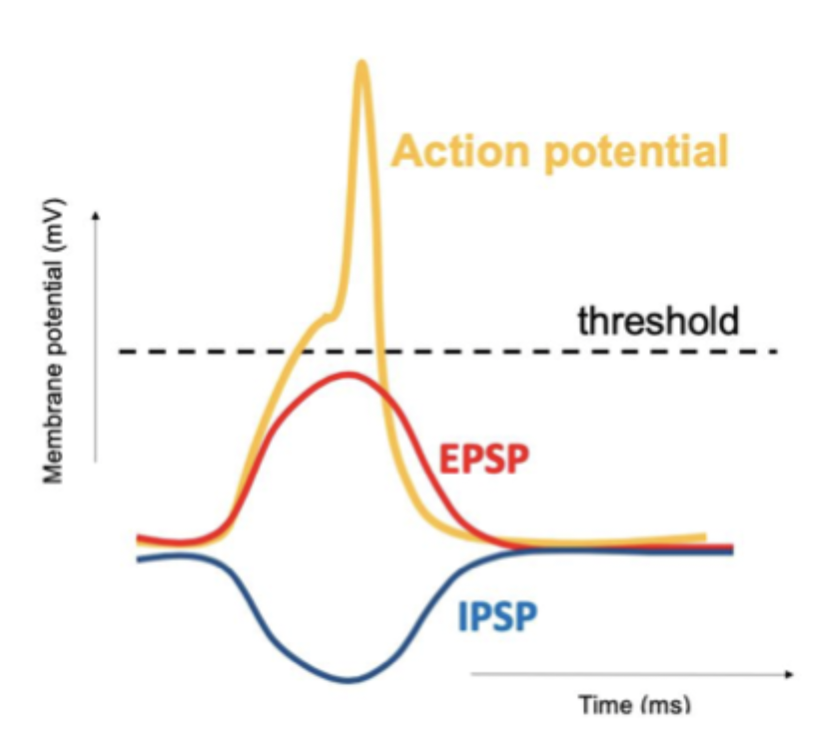
IPSPs and EPSPs
neurons receive both inhibitory (IPSP) and excitatory (EPSP) inputs at the same time which can summate
If majority of inputs open K+ channels MP will be pushed toward Ek, cell less likely to fire AP because K+ inflow is inhibitory
If majority of inputs open Na+ channels MP is more positive (depolarised) so AP more likely to fire
Neurons are always integrating negative and positive inputs
Threshold
depolarising local potentials= can open VG Na channels = further depolarisation
If sufficient Na channels at initial segment there could be large influx of Na (threshold)
initial segments charge is positive in the cell and segment 2 and 3's charge are negative in the cell.
Voltage goes towards Na equilibrium (+65)
Voltage gated Na+ channel
At rest channel is closed. When channel is opened for short period of time and inside is positive, pore is blocked and channel is inactivated. It is reopened/reactivated when the voltage is negative again.
Action potential sequence
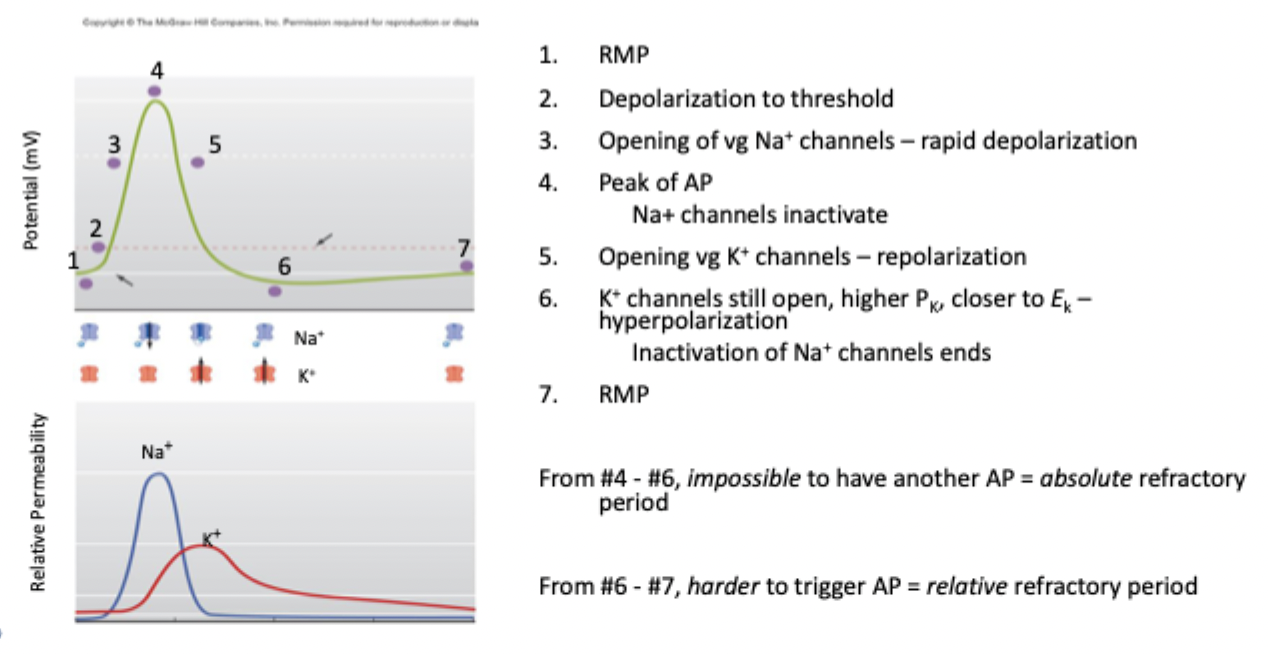
Absolute refractory period, relative refractory period
even with large stimulus AP can’t be generated because VG Na+ channels are inactivated
AP generated only if there is a very large stimulus because there high K+ conductance
Prevents AP from propagating backwards
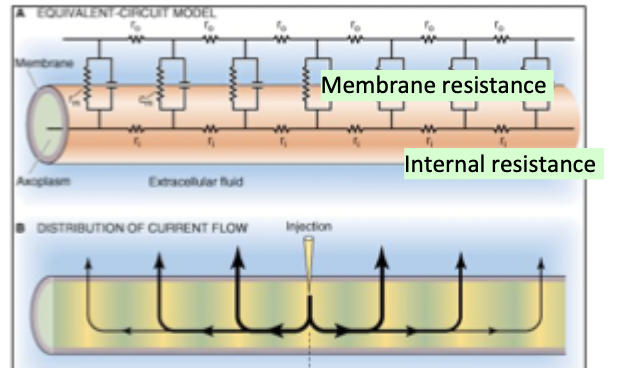
Excitable cells as electric circuits
If internal resistance is lower then electrotonic current will spread along the internal resistors.
If internal resistance is high then current will go up and out of the membrane causing it to not spread as far along the axon
For AP we want internal resistance to be low
How to make AP transmission faster
Bigger diameter= lower internal resistance= further electrotonic spread along axon= faster AP conduction
High transmembrane resistance (using myelin)
Myelinated vs unmyelinated axons
Fast saltatory conduction (jumps between nodes), high energy efficiency bc less ion exchange at nodes, has nodes of ranvier, goes up to 120m/s
slow continuous conduction, low energy efficiency bc more ions pumping along axon, no nodes, 0.5-2m/s
Hypokalemia vs Hyperkalemia
Low ECF K+, loss from digestive tract (vomiting, dieareah), loss of fluid from body. Reduced nerve & cardiac excitability bc MP is more negative. Causes weakness, fatigue, cramps, arrhythmia.
High ECF K+, inability to clear K+ (kidney disease), Addisons disease (low aldosterone), body can’t remove enough K+. Longer depolarisation because MP is more positive. Causes life threatening arrhythmia.
Things that block AP
Anesthetics= Blocking VG Na channels would stop the AP generation and therefore stop the pain signals from being perceived.
Toxins: bind ion channels and hold them open or closed
Tetrodotoxin and saxitoxin blocks Na channels but can't unblock them so AP can't be generated so kills the person.
Batrachotoxin holds Na channels open, this causes the membrane potential to go towards Na equilibrium potential but can't be polarized because of the high sodium entry into the cell.
Dendrotoxin= blocks voltage gated K channels so membrane can't be repolarized after AP so action potentials can't be fired.
p100
average waveform that represents average latency (100ms) for a visual evoked potential
what causes delay between stimulus and p100
Phototransduction, signal transmission and synapses
Cortical evoked potenial
electrical response to specific stimulus
Typical amplitude of nerve AP
AP is 100x bigger than EEG waveform. EEG only records postsynaptic events in superficial cortical regions.
why us WNP biphasic
APs propagate at different speeds along different axons, travels like a wave
Rat sciatic nerve
Increasing stimulation caused more axons within sciatic nerve to be activated, all-or-nothing response doesn’t occur
Individual axons (single axon response)
Sub threshold= Local depolarisation in axon, local potential
After sub threshold response (max response and above)= Action potential, all or nothing, same amplitude every time
Whole nerve response
Sub threshold stimulus= no response
At threshold there are first signs of WNP
WNP increases as stimulus increases
WNP is at max when all axons within nerve are activated
Refractory period, absolute refractory period,
period of reduced excitability after AP
another stimulus can’t create 2nd AP
Inactive gate of VG Na+ channels
What conditions change condition velocity in exvivo rat nerve
outside the animal
Temperature, handling, preparation, age, environment of nerve
How does myelination improve conduction velocity of individual axons
Electrical insulation ensures reduced leakage which makes conduction propagate further and increases transmembrane resistance. Myelinated axons have less axonal resistance.
Lignocane vs deadly toxins
Binds to VG Na+ channels and stops them from opening, temporarily blocks AP, low affinity block, reversible
Permanently stops AP which causes death, high affinity block, not reversible