TopHat Chem Ch8: Non-covalent interactions
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Match the following items.
I- Polar
II- Polar
III- Nonpolar
IV- Polar
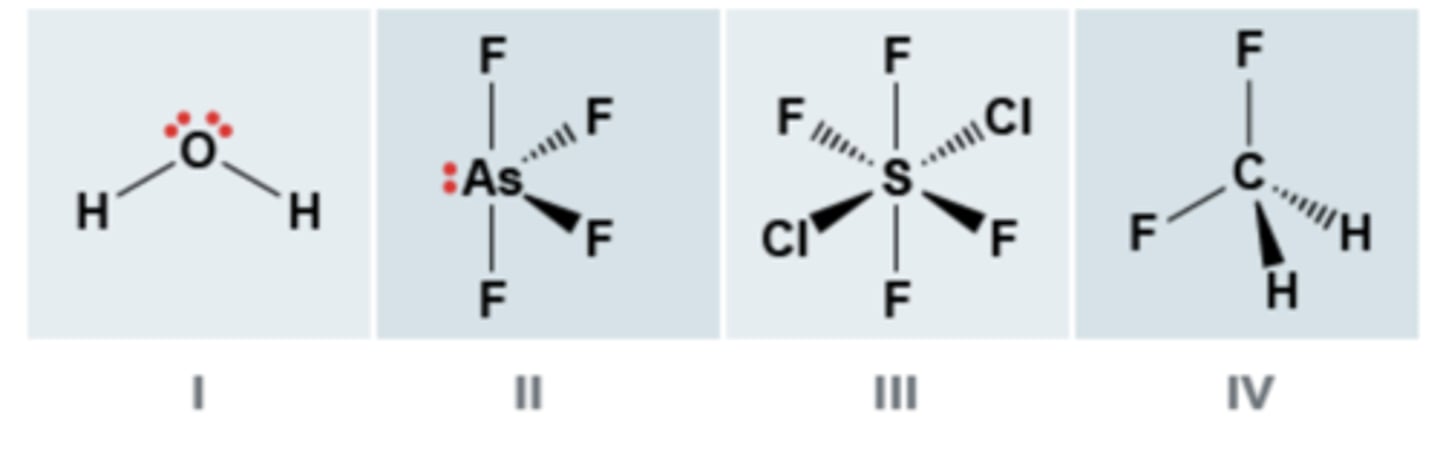
Classify each of the molecules below as polar or non-polar.
I- Polar
II- Nonpolar
III- Polar
IV- Nonpolar
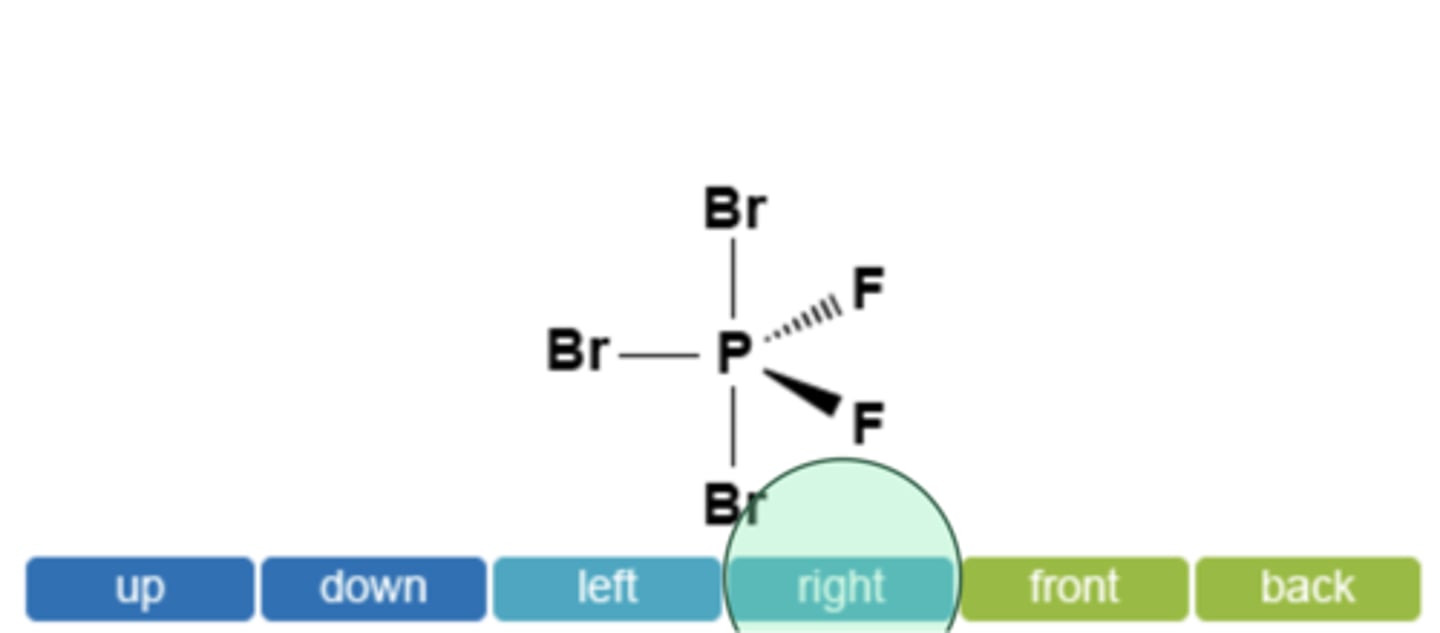
Which direction would you expect the dipole moment for PBrF to be oriented? You may click on multiple boxes if necessary.
right
Shown below is a molecular representation of the reaction given in Equation 10.1. Both the intramolecular and intermolecular forces involved in this reaction are illustrated with either lines or dashes. Which of the two forces, intramolecular or intermolecular, are represented by the dashes?
Intermolecular
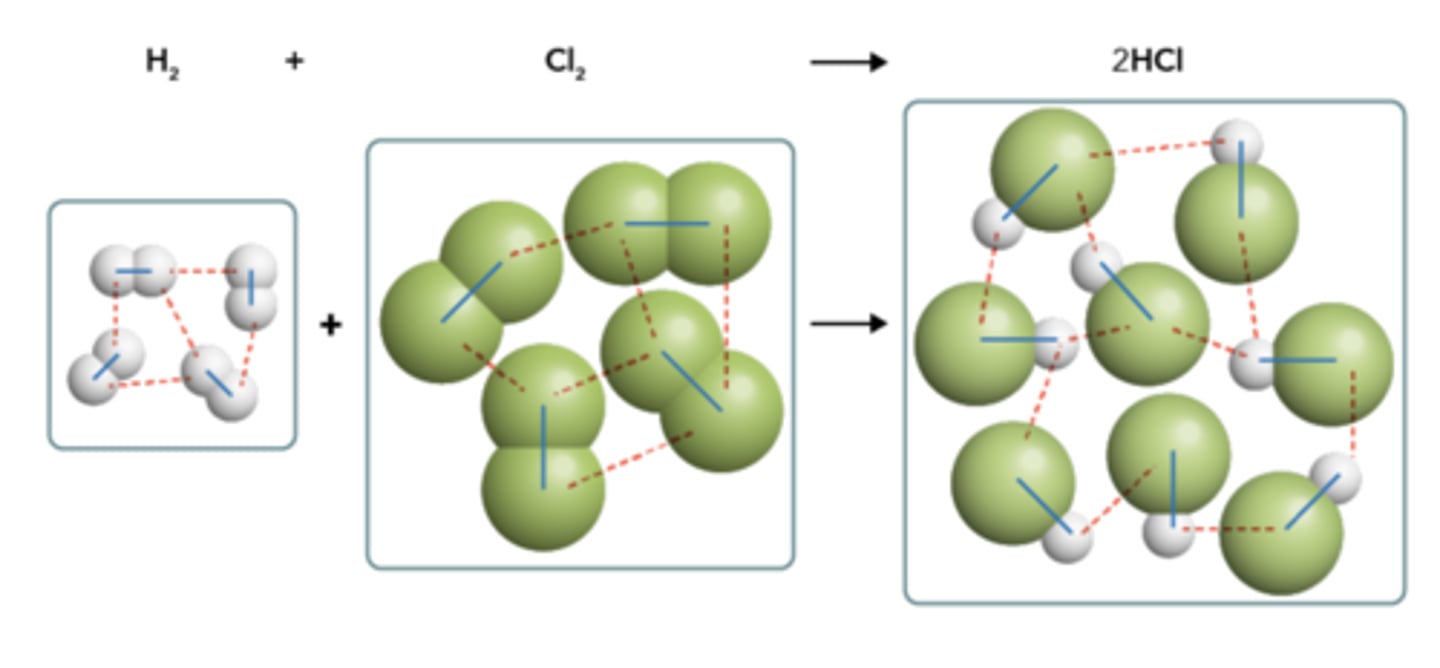
Consider the molecular formulas below and classify the following compounds as ionic, covalent, or a mixture of ionic and covalent.
CaCO3- Mixture
NH4NO3- Mixture
SOCl2- Covalent
LiF- Ionic
Use the electronegativity values from Table 10.2 to determine if the following bonds are likely to be non-polar covalent, polar covalent, or ionic. Match each bond to its appropriate classification.
Li-C: Polar covalent
H-C: Non-polar covalent
O-C: Polar covalent
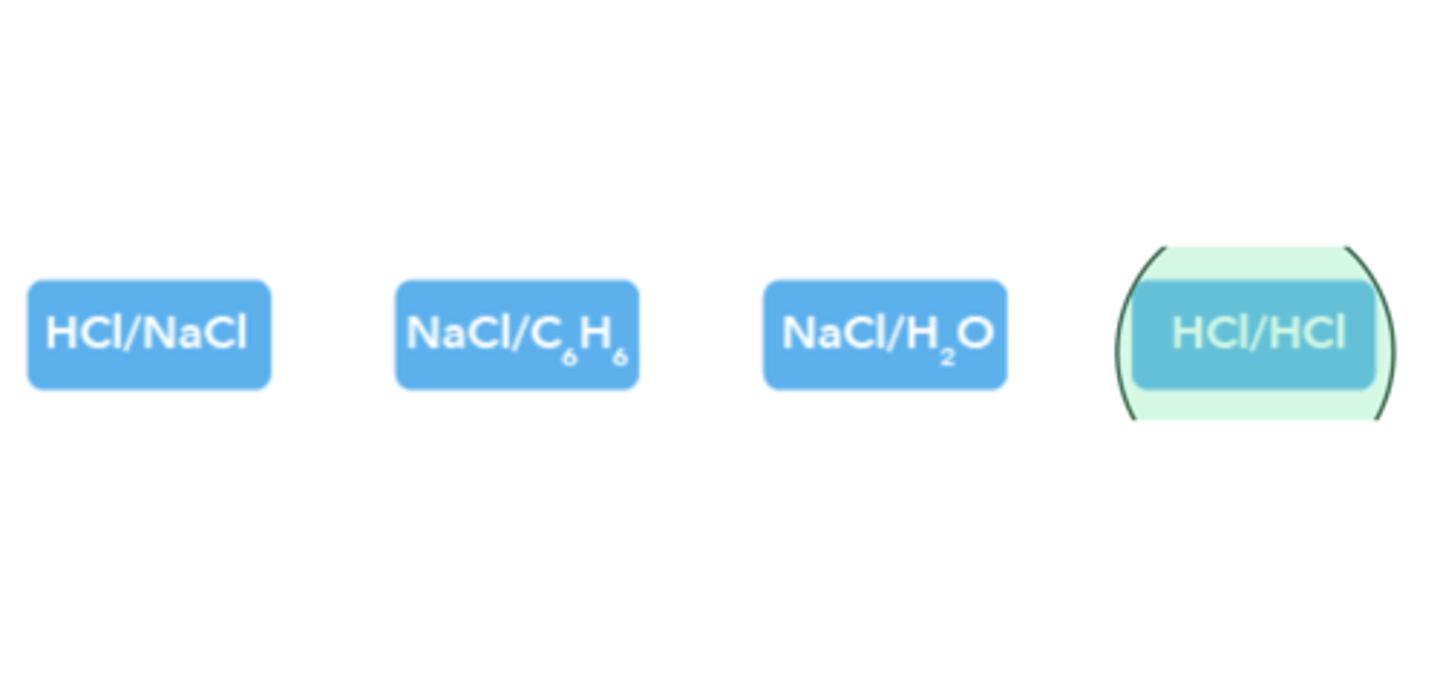
Which of the following pairs of molecules have dipole-dipole forces between them?
HCl/HCl
Arrange the following in order of increasing dipole-dipole interaction. List the molecule with the least dipole-dipole interaction at the top.
CH4
CH3I
CH3Cl
CH3F
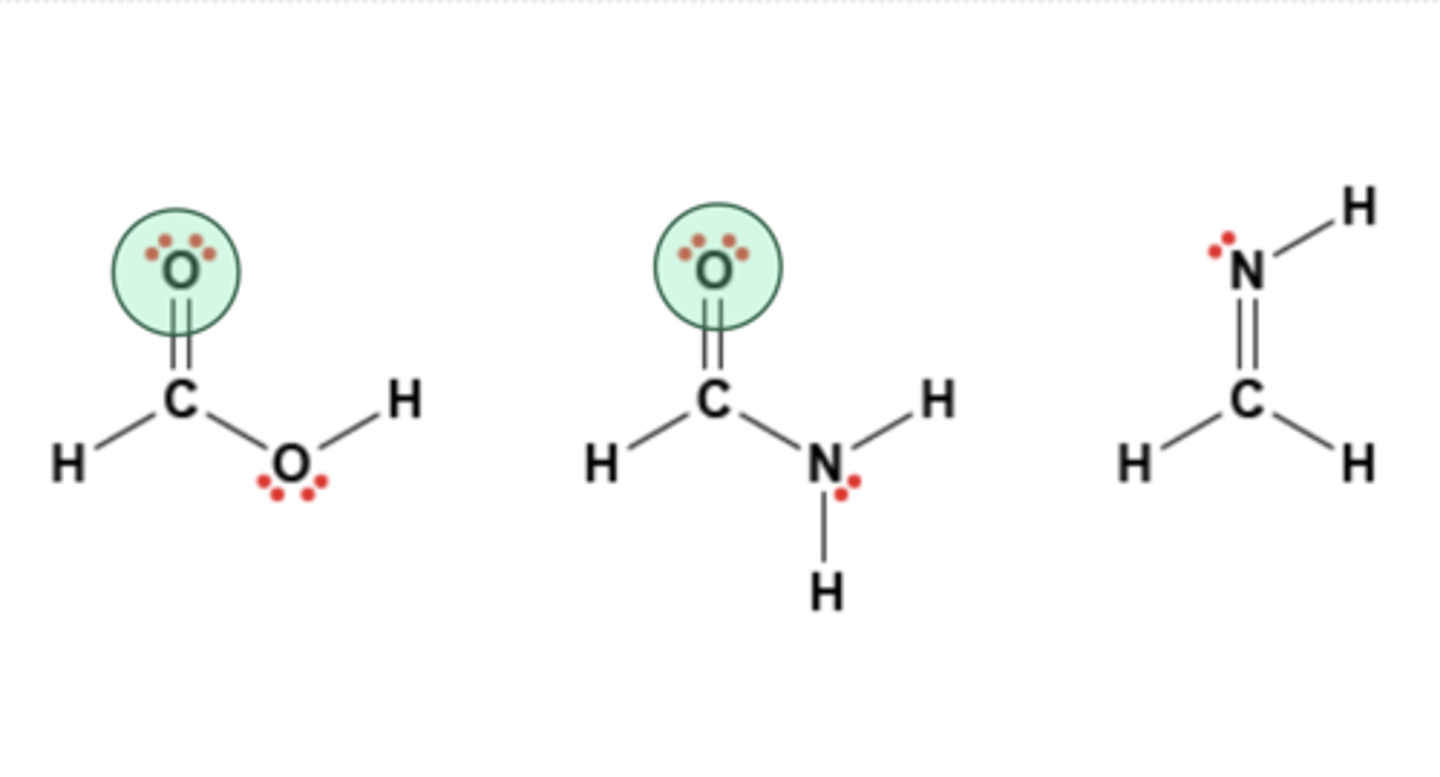
Shown below are the molecular structures of three molecules. Which atoms on these molecules are hydrogen bond acceptors only?
shown in picture
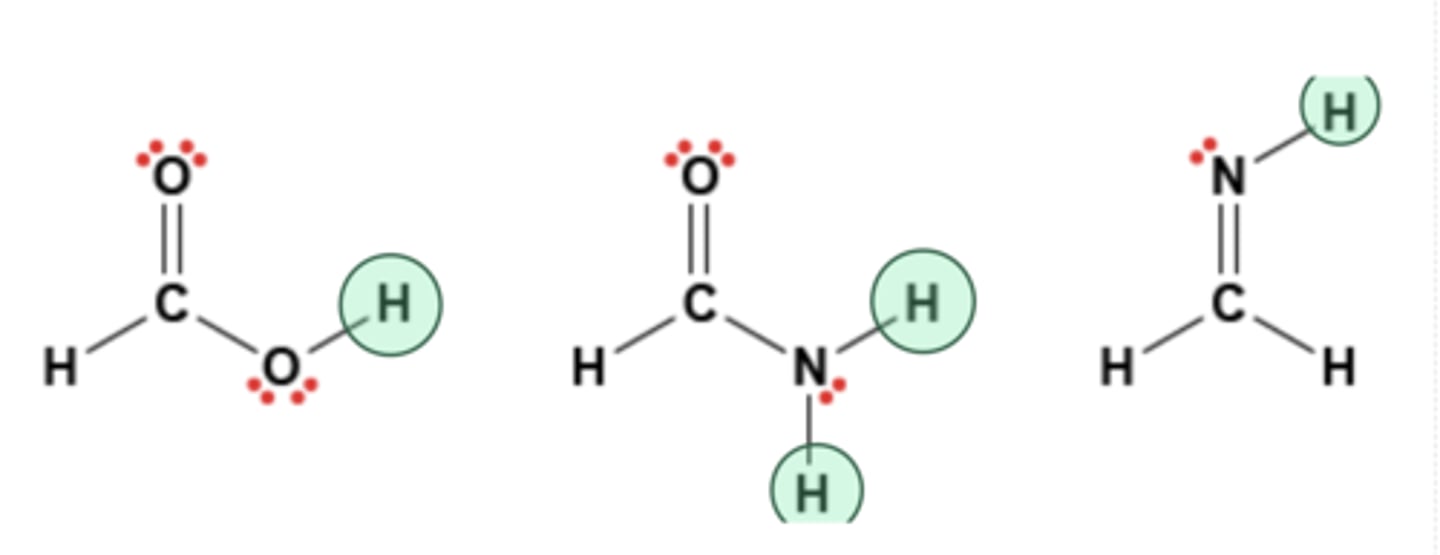
Shown below are the molecular structures of three molecules. Which hydrogen atoms on these molecules could participate in hydrogen bonds?
shown in picture
Which of the following substances does not have ion-dipole forces? Note: (aq) means an aqueous solution (i.e. in water) and (s) means solid (i.e. no water around).
KCl(aq)
NaCl(aq)
CaCl2(s)
None of them
CaCl2(s)
Which of the following mixtures/compounds have ion-dipole forces between them?
HCl/H2O
NaCl/C6H8
NaCl/H2O
pure H2O
NaCl/H2O
Choose the correct intermolecular force from Column B that matches the compound given in Column A.
Liquid NH3: hydrogen bonding
Aqueous KCl: ion-dipole
H3C-O-CH3: dipole-dipole
Match the substances given in Column A with the correct intermolecular forces shown in Column B. (Hint: You will need to draw Lewis dot structures.)
CH3OH: London dipole-dipole, and Hydrogen bonding
CBr2Cl2: London dispersion and Dipole-Dipole
CH3NH2: London dispersion dipole-dipole and Hydrogen bonding
BH3: London dispersion
BeCl2: London dispersion
CH3OCH3: London dispersion and Dipole-Dipole
Select all intermolecular forces experienced between molecules in a pure sample of the following compound.
London dispersion
Dipole-dipole
Hydrogen bonding
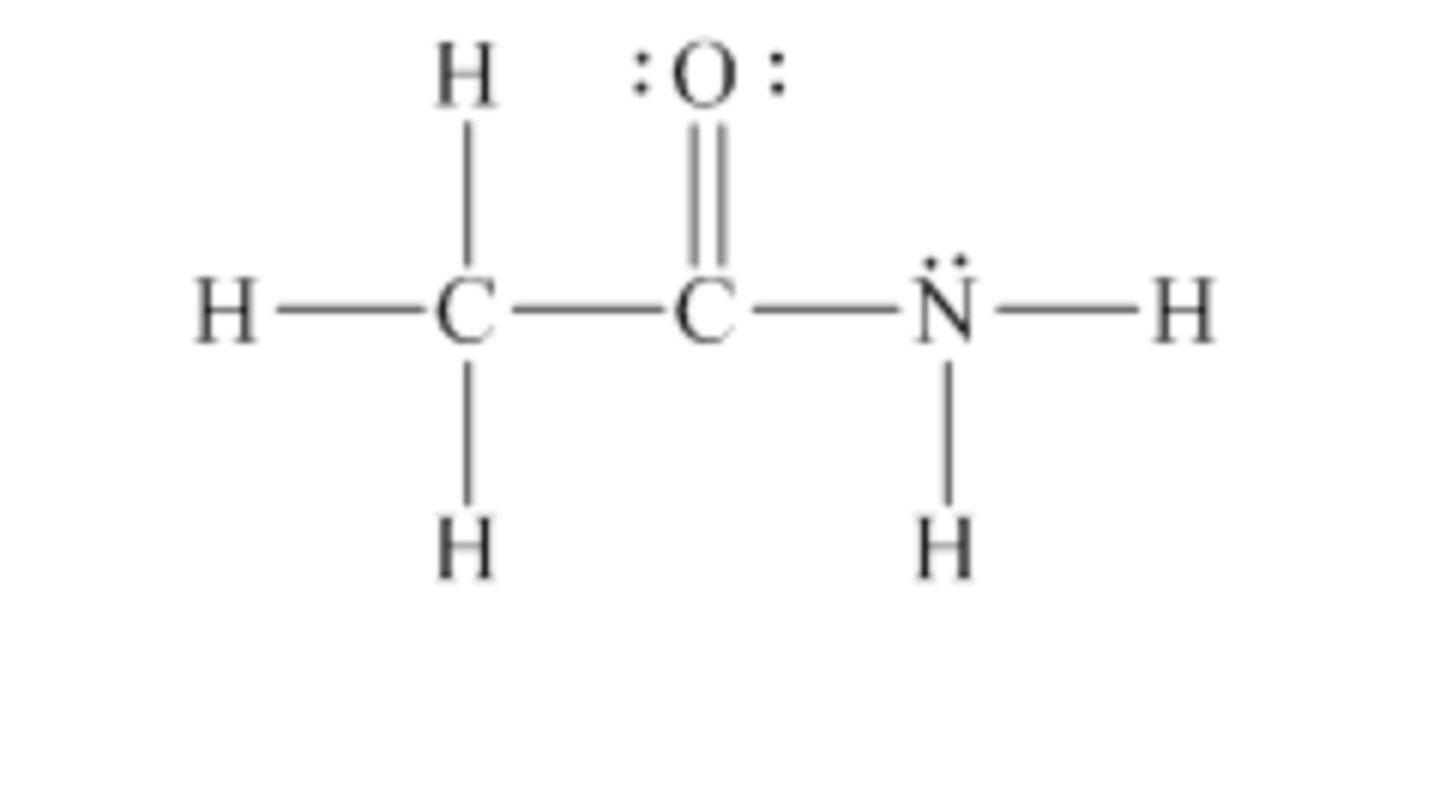
Select the dominant intermolecular force experienced between molecules in a pure sample of the following compounds. Remember that lone electron pairs are not drawn.
Dipole-dipole
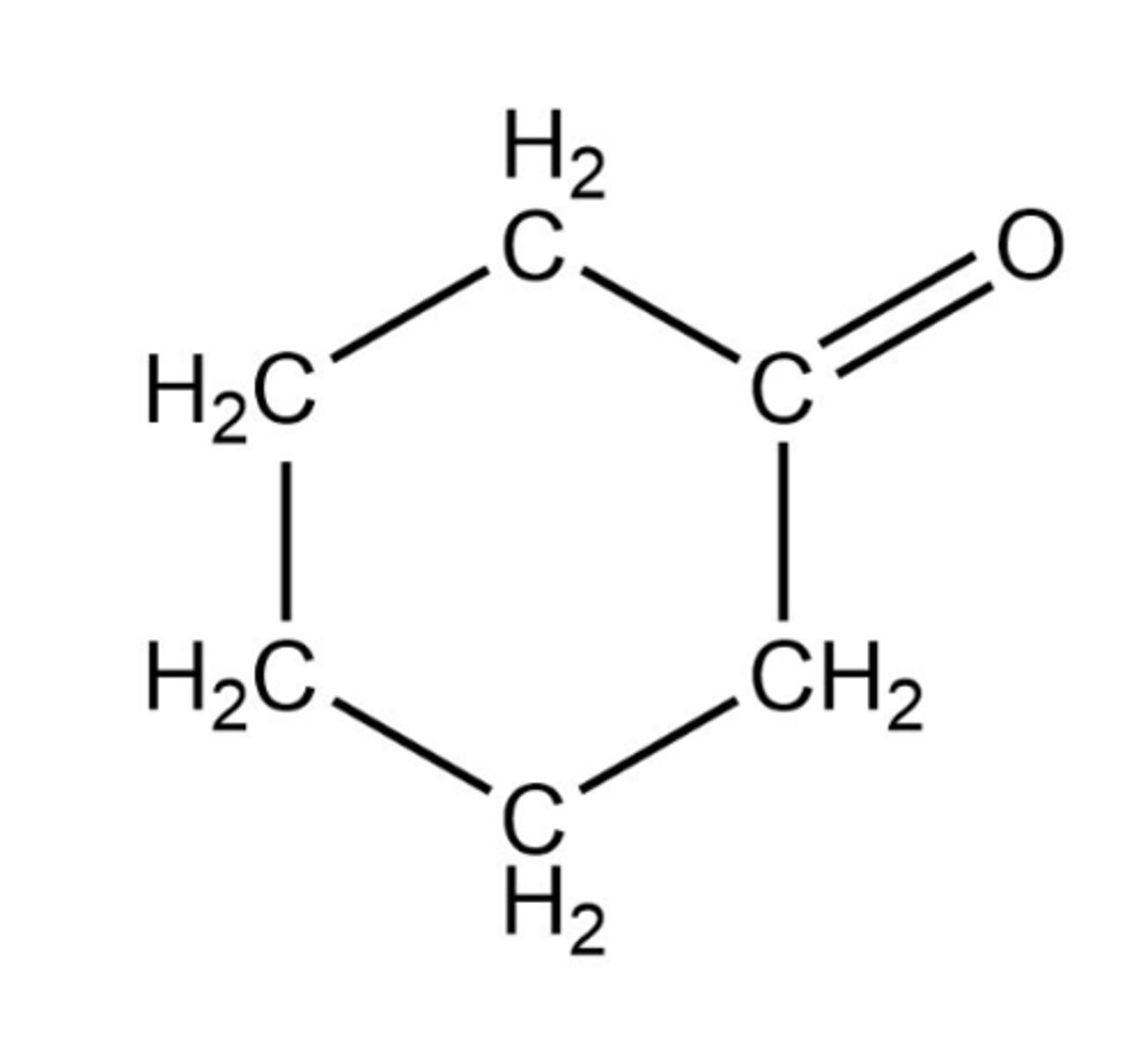
Select the dominant intermolecular force experienced between molecules in a pure sample of the following compounds. Remember that the lone electron pairs are not drawn.
Hydrogen bonding

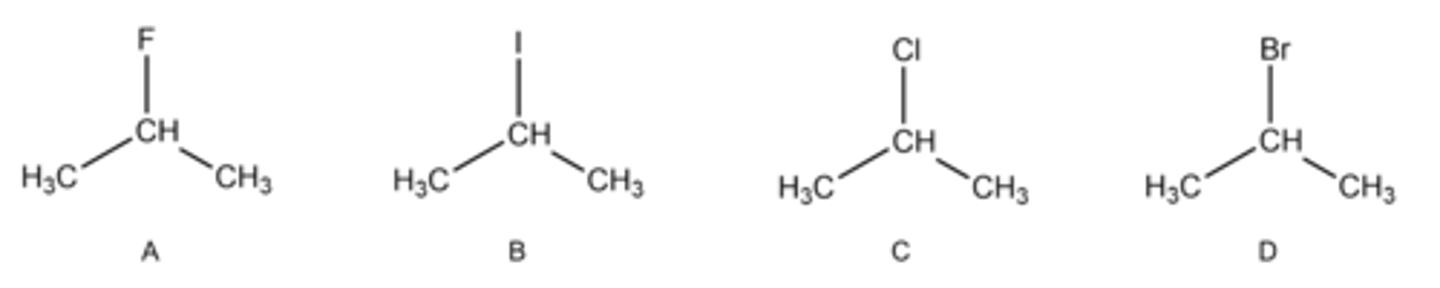
Choose the molecule that would have the strongest London dispersion forces. Remember that lone electron pairs are not drawn.
B
Which of the following substances has the highest boiling point?
H2
N2
O2
Xe
Xe
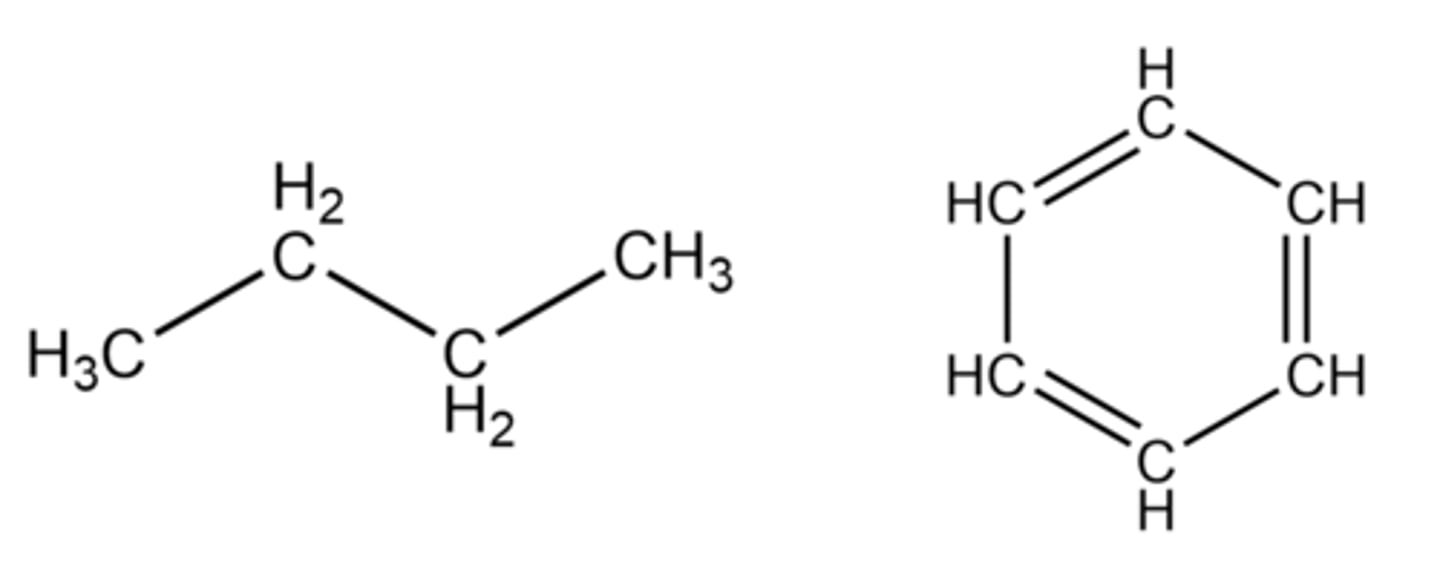
Shown in the figure below are the molecular structures of three compounds that all have the molecular formula CH. Arrange these substances according to increasing boiling point (lowest boiling point on the top and highest boiling point on the bottom).
Top left
Bottom
Top right
Do you expect these substances to mix?
High misciblity
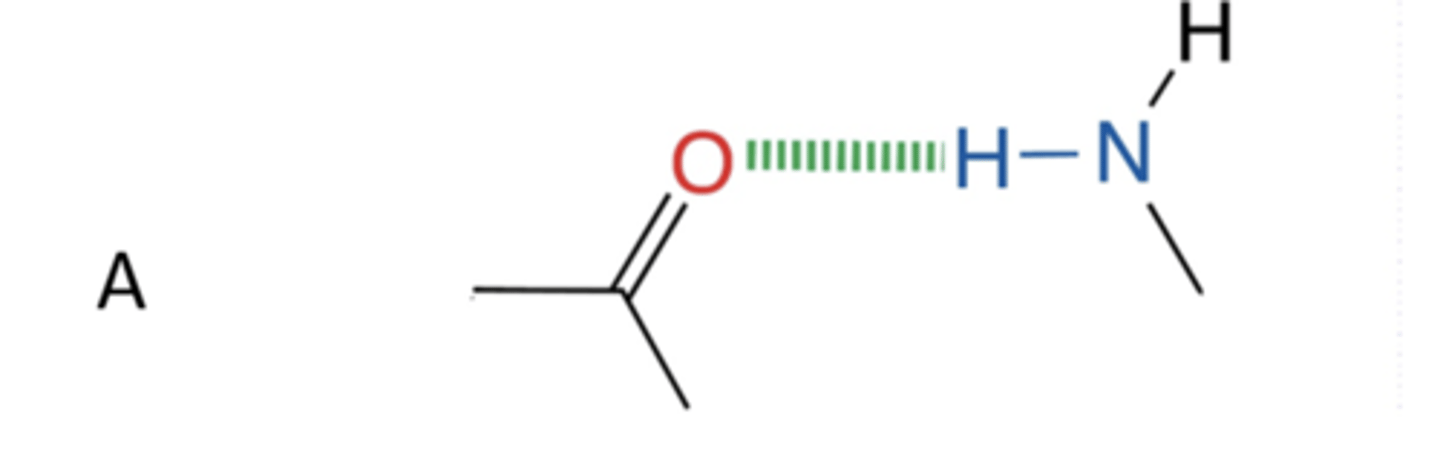
Which non-covalent interactions (dashed lines) are occurring between the molecules shown in panel A of the figure above?
hydrogen bonding
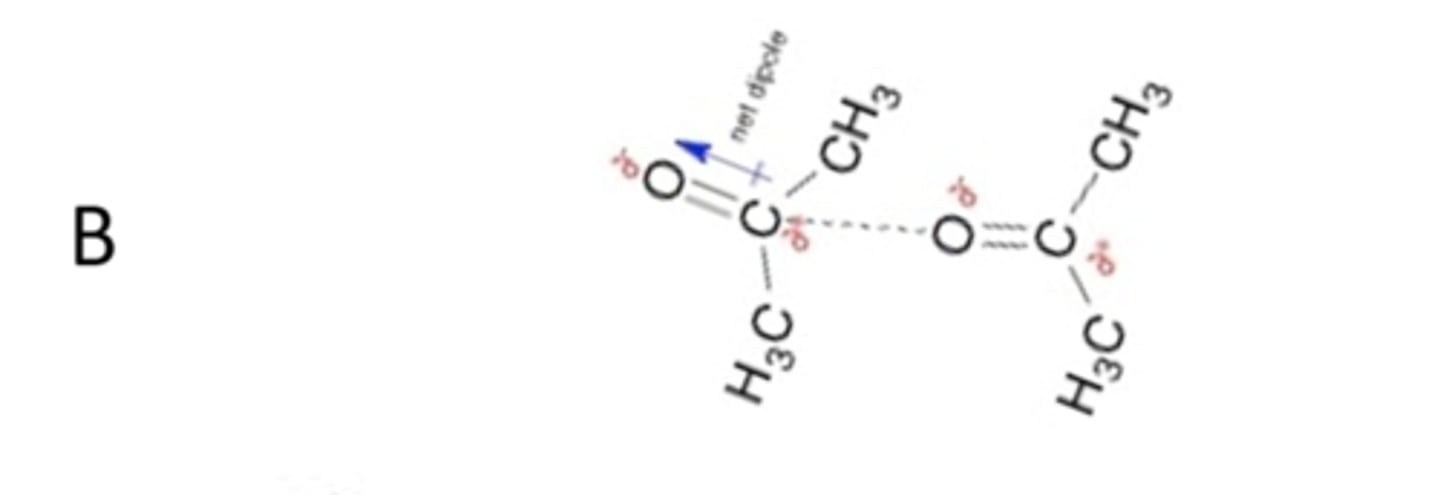
Which non-covalent interactions (dashed lines) are occurring between the molecules shown in panel B of the figure above?
dipole - dipole interactions
Which non-covalent interactions are occuring between the hydrocarbon chains (just carbon and hydrogen) of the lipid molecules shown in panel C (a section of a cell membrane) of the figure above?
dispersion interactions

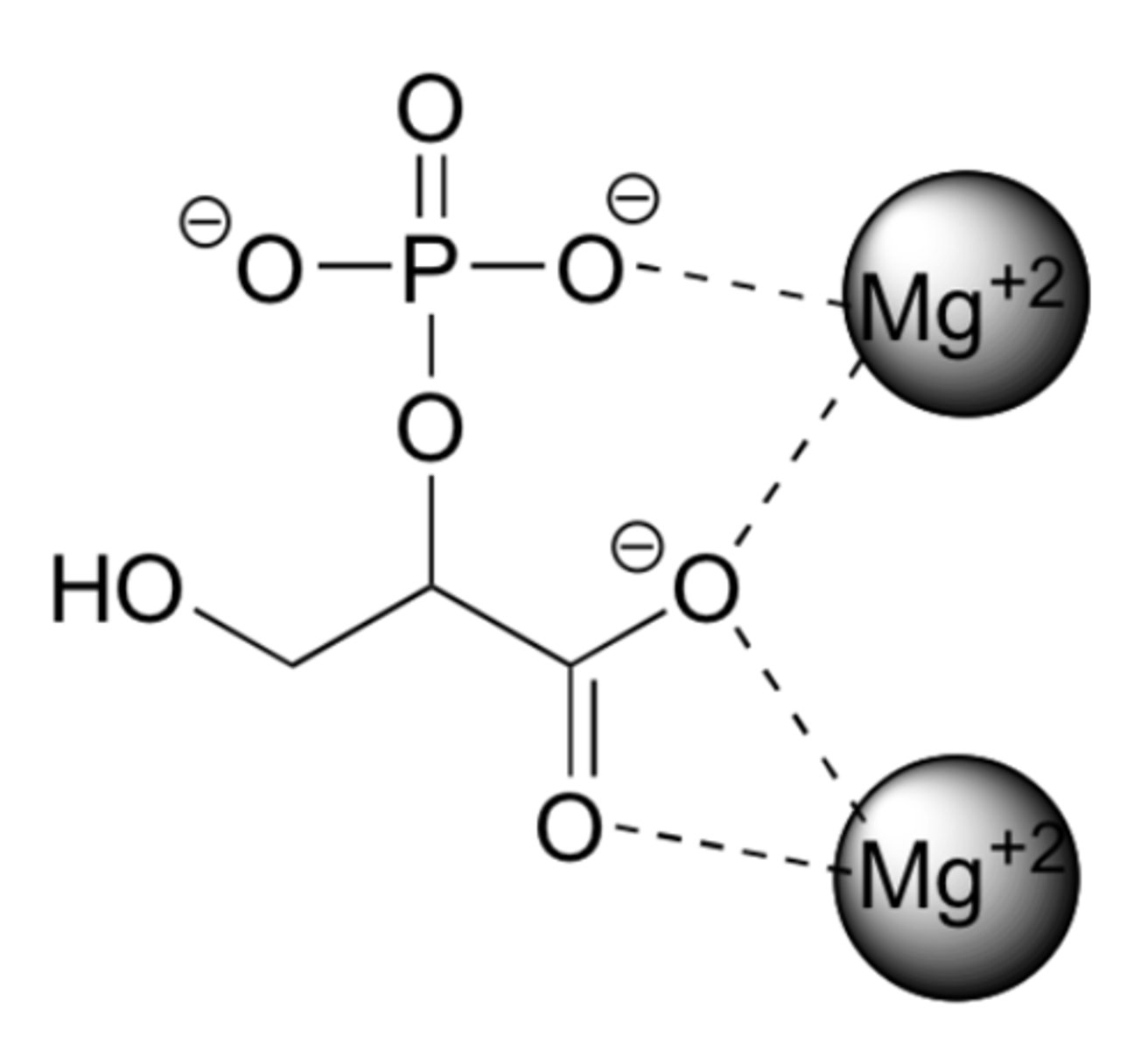
In this figure, 2-phosphoglycerate, an intermediate in the glycolysis pathway, interacting with two Mg+2 ions in the active site of a glycolytic enzyme called enolase is shown. These interactions are essential for glycolysis. What is the strongest non-covalent interaction occurring to stabilize the complex.
ion - dipole interactions
Which molecule will be more soluble in water and why?
Ethanol because it is a hydrogen bond donor and acceptor and, therefore can form three hydrogen bonds with water
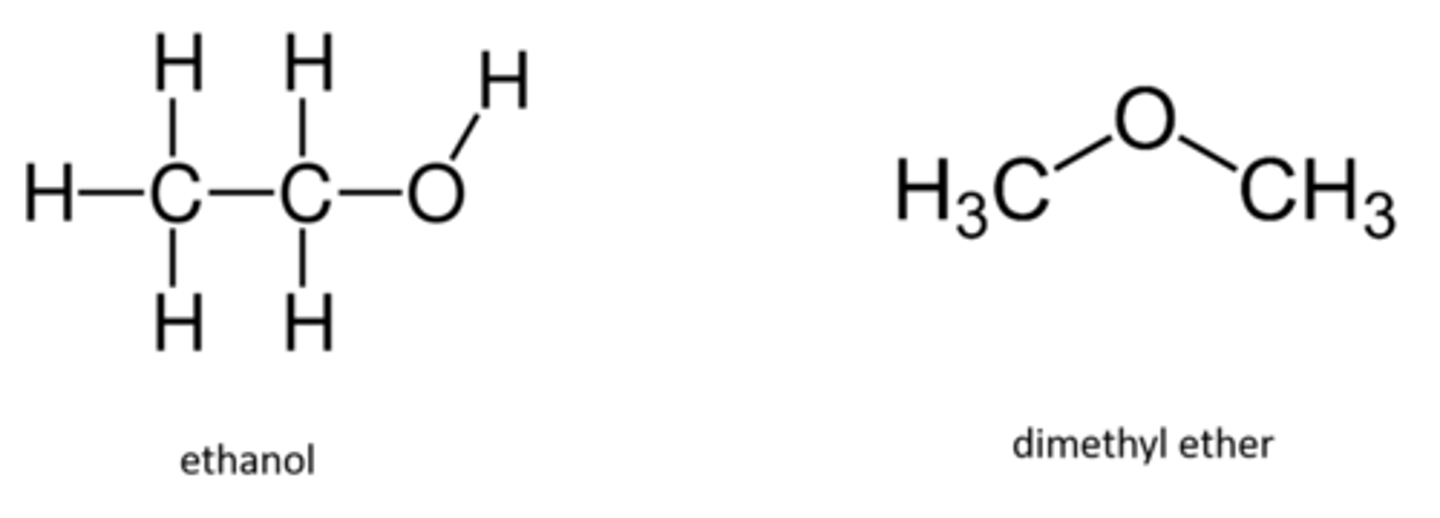
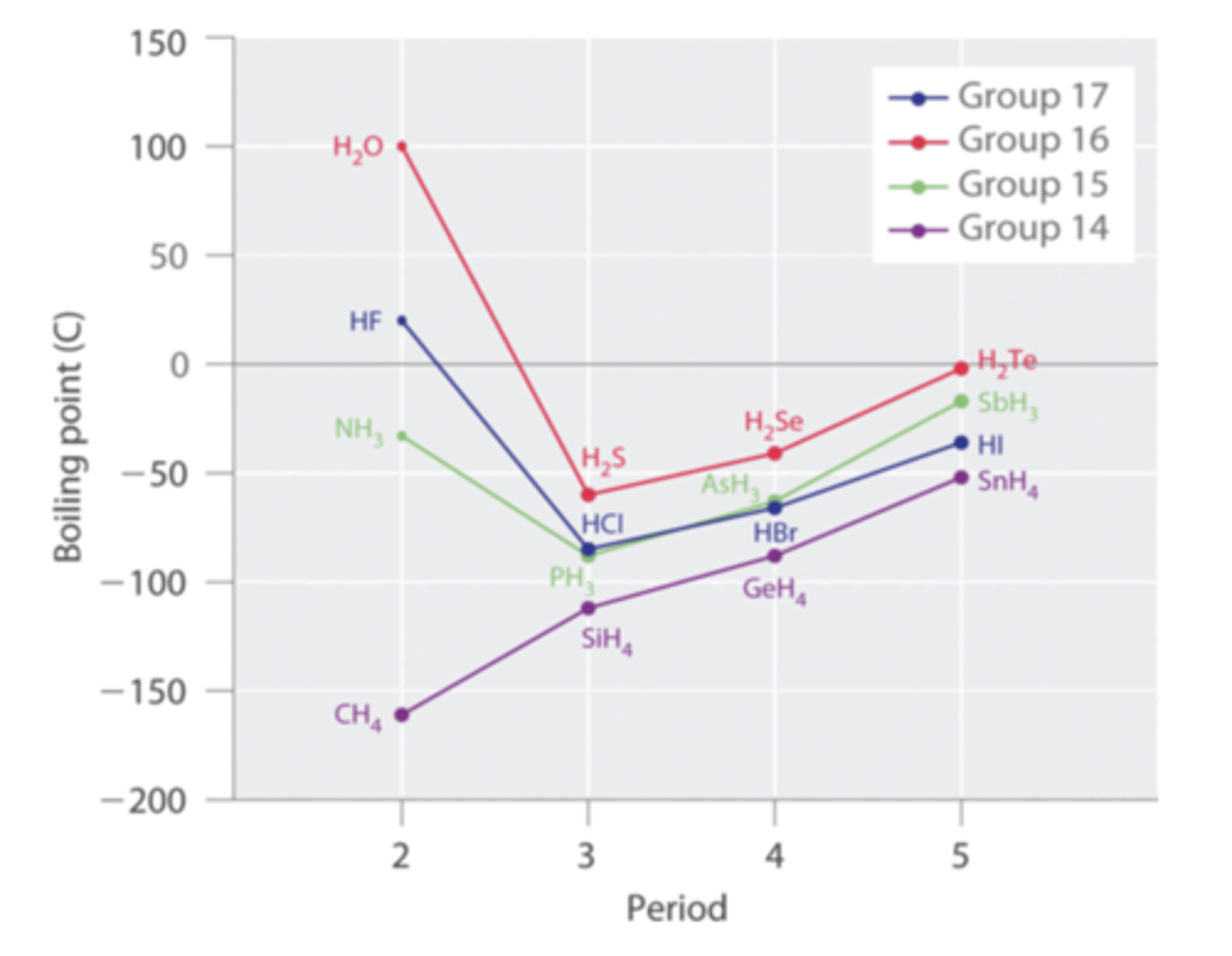
The graph below is similar to the one discussed in CH09 Video 1 with additional molecules plotted. Why does NH3 have a higher boiling point than PH3 but CH4 has a lower boiling point than SiH4?
Dispersion forces are weak forces that increase in strength with the size of the molecule. CH is smaller than SiH and, therefore, has weaker dispersion forces attracting the molecules together so it takes less energy (lower boiling point) to break the CH molecules apart than the SiH molecules apart. Although NH is smaller than PH, there is an additional forces that is stronger than dispersion forces attracting the NH molecules that the PH molecules don't have. NH can form hydrogen bonds with itself increasing the attraction of the molecules and requiring more energy (higher boiling point than PH, which does not form hydrogen bonds.
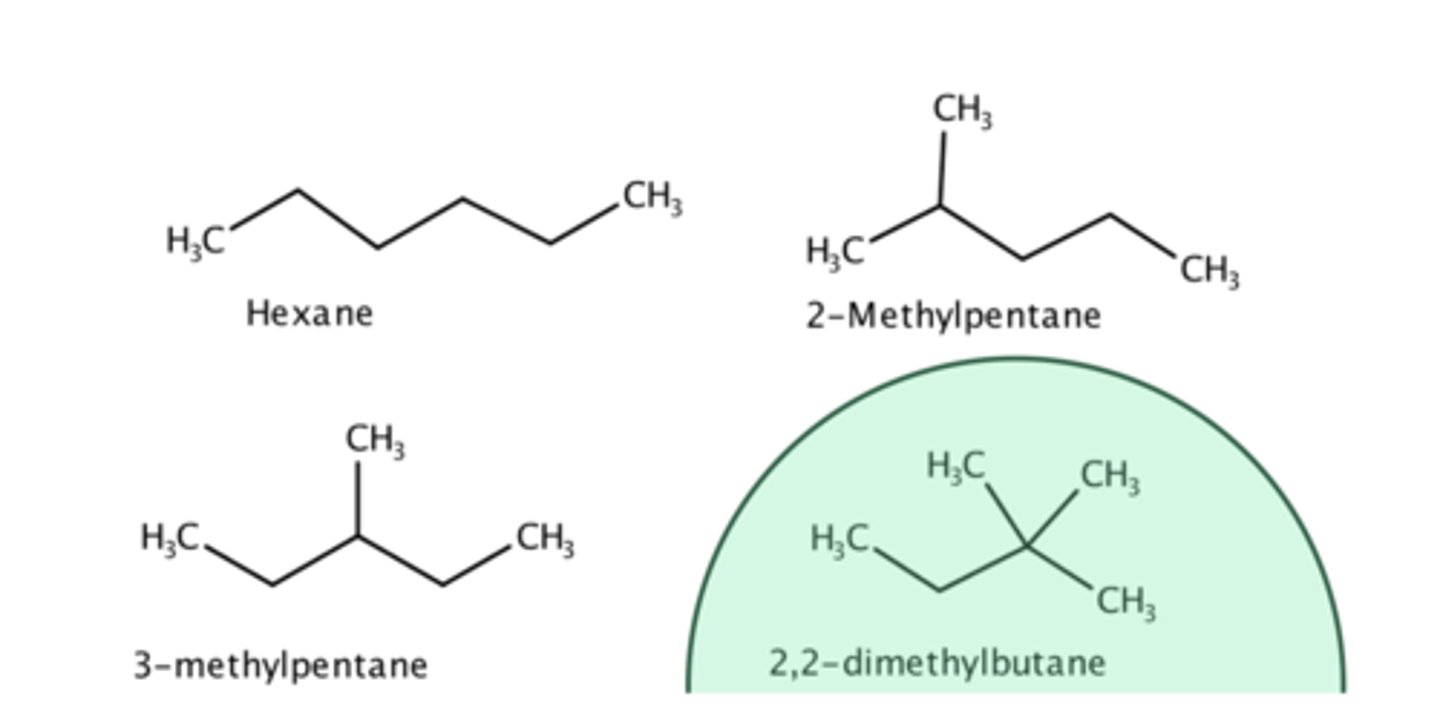
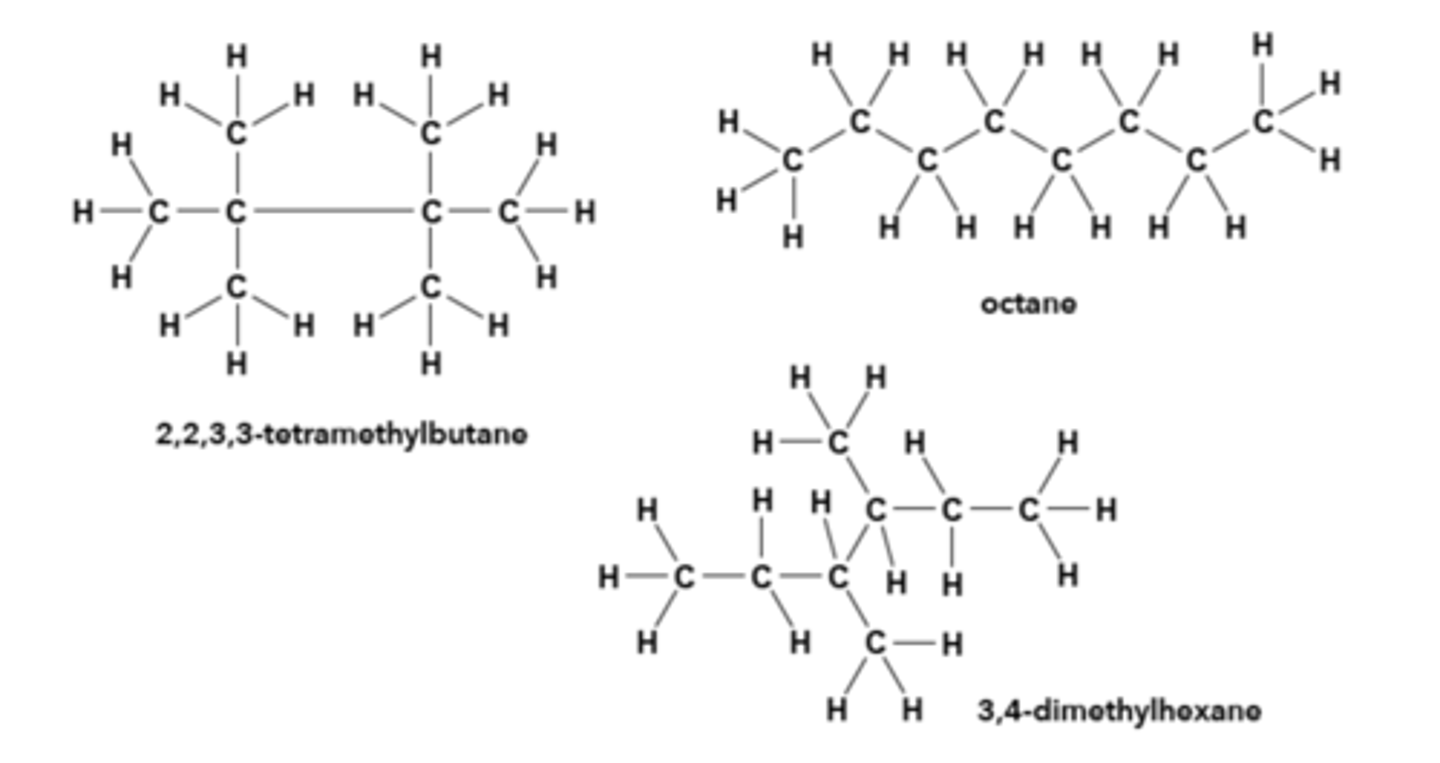
Click on the molecule that has the lowest boiling point.
dimethylbutane