Chemistry - Properties and structure of atoms, trends in the PT
1/49
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
Solid Sphere Model
Proposed by John Dalton in the early 1800s; suggests that substances are made of small hard spheres called atoms that cannot be divided.
Plum Pudding Model
Modified by J J Thomson in the early 1900s; describes an atom as a sphere of positive charge with negatively charged electrons embedded within it.
Nuclear Model
Developed by Ernest Rutherford between 1909 and 1911; suggests that the mass of the atom is concentrated in the nucleus, which has a positive charge.
Planetary Model
Proposed by Niels Bohr in 1913; suggests that electrons orbit the nucleus in shells at set distances.
Atomic Model
Developed by James Chadwick in 1932; includes neutrons in the nucleus among protons and electrons.
Alpha Scattering Experiment
An experiment conducted by Ernest Rutherford that demonstrated the structure of the atom by firing alpha particles at gold foil.
Periodic Trends
The patterns observed in atomic properties such as atomic radius, ionization energy, and electronegativity across different elements.
Atomic Radius
The size of an atom; it depends on the number of electron shells and the number of protons in the nucleus.
Ionization Energy
The energy required to remove an electron from an atom.
Electronegativity
The measure of an atom's ability to attract and hold onto electrons.
Effective Nuclear Charge
The net positive charge experienced by valence electrons, calculated as the number of protons minus the number of inner electrons.
Trend (Atomic Radius)
Increases down a group and decreases across a period in the periodic table.
Shielding Effect
The phenomenon where inner-shell electrons reduce the effective nuclear charge felt by outer-shell electrons.
Patterns in Ionization Energy
Ionization energy increases across a period and decreases down a group due to changes in atomic size and effective nuclear charge.
Successive Ionization Energies
The energy required to remove each subsequent electron from an atom; these energies increase due to greater attraction between the nucleus and remaining electrons.
Electronegativity Trend
Electronegativity increases across a period and decreases down a group in the periodic table.
Trends in Properties Across a Period
As one moves across a period in the periodic table, atomic radius decreases, electronegativity increases, and ionization energy increases.
Trends in Properties Down a Group
As one moves down a group in the periodic table, atomic radius increases, electronegativity decreases, and ionization energy decreases.
Core Charge
The effective nuclear charge felt by the valence electrons, determined by the number of protons minus the shielding effect of inner electrons.
Valence Electrons
Electrons in the outermost shell of an atom, which are involved in chemical bonding.
Ionic Radius
The radius of an ion; it can differ from the atomic radius depending on the gain or loss of electrons.
Group 1 Elements
Elements known to have low first ionization energies, making them more reactive.
Group 18 Elements
Noble gases with complete outer shells, having high ionization energies and low reactivity.
Nuclear Charge
The total charge of the nucleus, equal to the number of protons.
Electron Affinity
The amount of energy released when an electron is added to a neutral atom.
Trends in Group Elements
Show consistent patterns across periods and groups regarding atomic properties such as size and reactivity.
Electronic Configuration
The distribution of electrons in an atom or ion, often represented by a series of numbers and letters that indicate energy levels and subshells.
Principal Energy Level
The main energy levels in an atom, denoted by the quantum number n, where n=1, 2, 3, 4, etc.
Orbitals
Regions in an atom where there is a high probability of finding electrons; each orbital can hold a maximum of 2 electrons.
Subshells
Divisions of electron orbitals that contain specific number of orbitals, categorized as s, p, d, and f.
Electron Filling Order
The sequence in which electrons occupy orbitals, starting from the lowest energy orbital first.
Energy Level Diagram
A visual representation of the arrangement of electrons in an atom, showing the principal energy levels and subshells.
Ion Electronic Configuration
The electronic configuration of an ion which shows how electrons are arranged in the ion compared to the neutral atom.
Hund's Rule
A principle that states that electrons will occupy degenerate orbitals singly before pairing up. Electrons will occupy degenerate orbitals singly first, to maximize total spin.
Orbital Diagrams
Visual representations of electron configurations showing the distribution of electrons in orbitals.
Atomic Structure
Refers to the composition and arrangement of protons, neutrons, and electrons in an atom.
Nucleus
The very dense core of an atom that contains most of its mass, made up of protons and neutrons.
Electron Cloud
The region around the nucleus where the electrons are likely to be found; its mass is negligible compared to the nucleus.
Electrostatic Attraction
The force that holds the atom together, derived from the attraction between the negatively charged electron cloud and the positively charged nucleus.
Mass Number (A)
The sum of protons and neutrons in an atom's nucleus; represented as A = Z + N.
Atomic Number (Z)
The number of protons in the nucleus of an atom, which determines the element's identity.
Neutron Number (N)
The number of neutrons in an atom's nucleus.
Isotopes
Atoms of the same element that have the same number of protons but different numbers of neutrons, leading to different mass numbers.
Notation of Isotopes
Two common ways to write isotopes include the element's name followed by the mass number (e.g., Carbon-12) or the element's symbol with the mass number as a superscript (e.g., 12C).
Hydrogen Isotopes
Hydrogen-1 (mass number: 1), Hydrogen-2 (deuterium, mass number: 2), and Hydrogen-3 (tritium, mass number: 3).
Isotope Calculations
formula : •(Ar = %A x Ar(A) + %B x Ar(B) + %C x Ar (C)… etc) /100
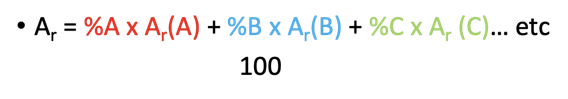
Relative Atomic Mass Calculation
A process to determine the average atomic mass of an element by factoring in the masses and relative abundances of its isotopes.
Abundance Calculation
The process of determining the percentage abundance of isotopes in a sample.
Copper Isotopes
Copper exists as Cu-63 and Cu-65, with relative abundances that can be calculated given the average atomic mass.
Lithium Isotopes
Lithium has two naturally occurring isotopes, 6Li and 7Li, with specific masses and their natural abundances can be calculated.