Unit 1 Quiz 1
5.0(1)
Card Sorting
1/21
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
1
New cards
Compounds/Molecules
Combination of 2 or more atoms formed by bonds
2
New cards
Ionic Bond
Transfer of electrons from one element to another, between a metal and nonmetal
3
New cards
Covalent Bond
Sharing of electrons between nonmetals to fill their valence orbital
4
New cards
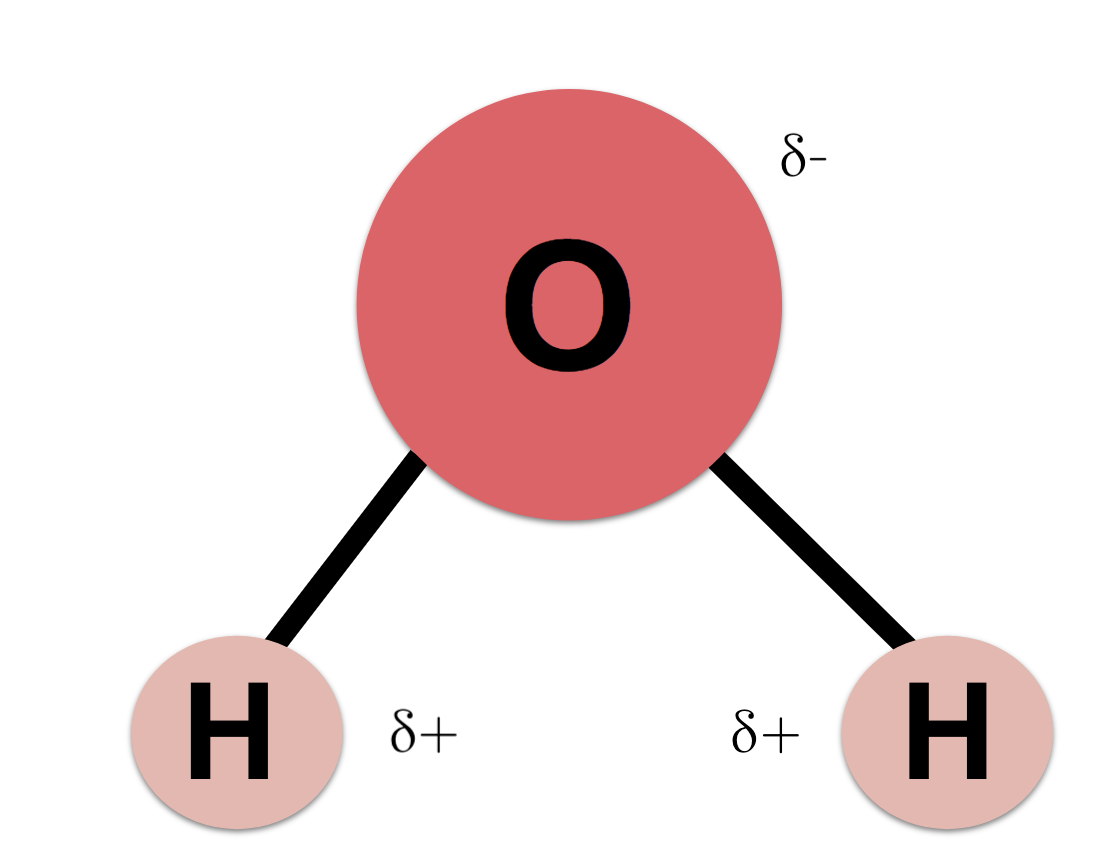
Water Structure
H2O, bent molecule with polarity and hydrogen bonds
5
New cards
Cohesion
Ability of water molecules to stick together
6
New cards
Adhesion
Ability of water to stick to other surfaces, forming hydrogen bonds
7
New cards
Solvent
Ability of water to dissolve other polar/charged molecules
8
New cards
Density of Water
Less dense at freezing, most dense at 4 degrees Celsius
9
New cards
Specific Heat of Water
High specific heat makes it difficult to warm and cool down water
10
New cards
pH
Measurement of hydrogen ions and hydroxide ions in a solution
11
New cards
Acid
Contains more hydrogen ions, pH range of 1-6.9
12
New cards
Base
Contains more hydroxide ions, pH range of 7.1-14
13
New cards
Neutral
Equal number of hydrogen ions and hydroxide ions, pH of 7 (water)
14
New cards
Proton
Positively charged in nucleus of atom.
15
New cards
Electron
Negatively charged orbiting atom, weighing near nothing.
16
New cards
Neutron
Neutrally charged in the nucleus of an atom.
17
New cards
What is an isotope?
An isotope is a variant of an element that has the same number of protons but a different number of neutrons in its nucleus.
18
New cards
What is the potential energy of the different shells around an atom?
The potential energy of shells around an atom increases with distance from the nucleus. The K shell has the lowest energy, followed by the L shell. The outer shells (M, N, O) have higher energy due to weaker attraction from the nucleus. Energy increases away from the nucleus.
19
New cards
polar covalent bonds
Type of chemical bond where electrons are unequally shared between atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other.
20
New cards
Non-Polar Covalent Bonds
Type of chemical bond where electrons are shared equally between two atoms, resulting in a balanced distribution of charge.
21
New cards
Cation
A positively charged ion formed by losing electrons.
22
New cards
Anion
A negatively charged ion formed by gaining electrons.