Principles of Chemistry II Exam 1
1/27
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
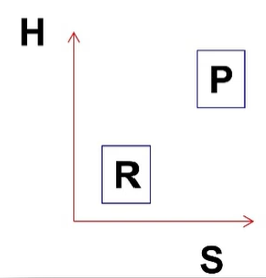
what is the H and S? PF or RF at what temperature?
H>0 S>0, PF at high temp
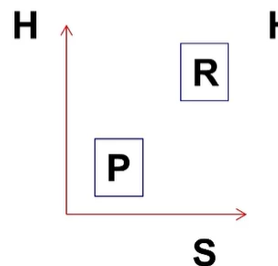
what is the H and S? PF or RF at what temperature?
H<0 S<0, PF at low temp
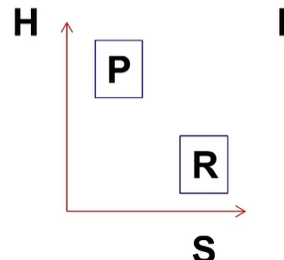
what is the H and S? PF or RF at what temperature?
H>0 S<0, never PF
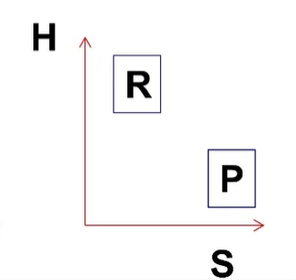
what is the H and S? PF or RF at what temperature?
H<0 S>0, always PF
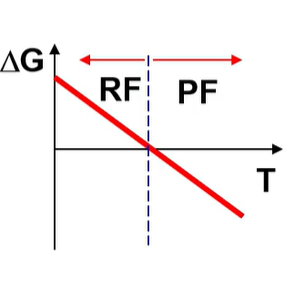
what is the H and S of this?
H>0 S>0
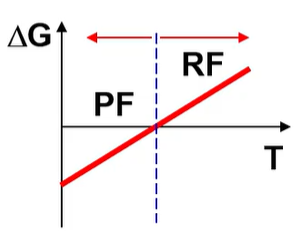
what is the H and S of this?
H<0 S<0
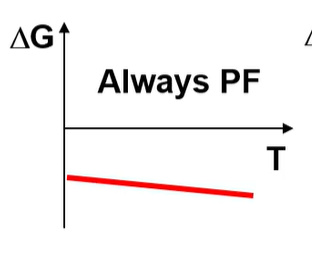
What is the H and S of this?
H<0 S>0
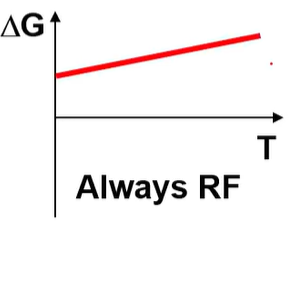
what is the H and S of this?
H>0 S<0
A negative delta g means?
a product favored reaction
A positive delta g means?
a reactant favored reaction
what is the equations for equilibrium constant?
products/reactants
A K>1 means?
A product favored reaction
A K<1 means?
A reactant favored reaction
the more O2 required during combustion of fuel means?
the more energy released
what do oxygenated fuels require and produce?
they require less o2 during combustion but also produce less energy per mole of fuel
does forming bonds is energy released or taken in?
released
when breaking bonds is energy released or taken in?
taken in
is breaking bonds and exo or endo thermic process?
endo because energy is taken in to break the bonds
how do elements get a standard enthalpy of Hf equal to zero?
it has to be in its standard base state, like C with graphite or O2 gas
what type of bonds will have a longer internuclear distance on the curve graphs?
double bonds or triple bonds, then A-B bonds, then A-A bonds
is more disorder good for entropy? (s)
yes it increases the entropy value of the compound
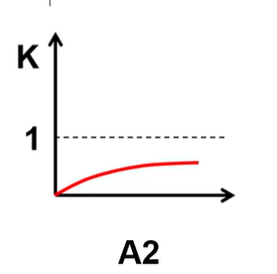
What is H and S of this and what is favored?
H>0 S<0 and RF
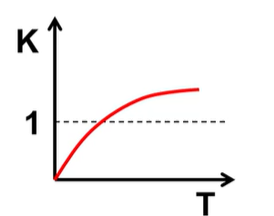
What is H and S of this and what is favored?
H>0 S>0 and PF at high T
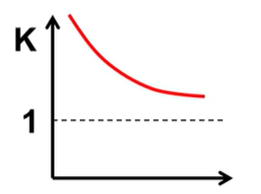
What is H and S of this and what is favored?
H<0 S>0 and PF
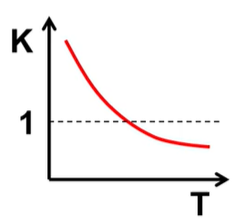
What is H and S of this and what is favored?
H<0 S<0 and PF at low T
how to decide which is more energetically stable?
solids, liquids, then gases then charges, the closer Fr the less stable because radius
if more bonds are broken than made it will be?
endothermic
if more bonds are made than broken it will be?
exothermic